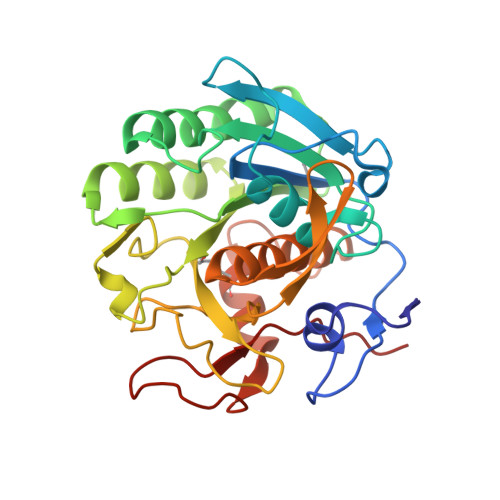
High-resolution structure of proteinase K cocrystallized with digalacturonic acid.
Larson, S.B., Day, J.S., Nguyen, C., Cudney, R., McPherson, A.(2009) Acta Crystallogr Sect F Struct Biol Cryst Commun 65: 192-198
- PubMed: 19255463
- DOI: https://doi.org/10.1107/S1744309109002218
- Primary Citation of Related Structures:
3DYB - PubMed Abstract:
Proteinase K, a subtilisin-like fungal protease, was crystallized from a cocktail of small molecules containing digalacturonic acid (DGA). The crystal structure was determined to 1.32 A resolution and refined to an R factor of 0.158. The final model contained, beside the protein, two calcium ions, 379 water molecules, a molecule of DGA and a partially occupied HEPES molecule. The DGA molecule has one sugar moiety disposed exactly on a crystallographic twofold axis; the second ring was not observed. The DGA molecule is bound to two protein molecules across the twofold axis through hydrogen-bonding networks involving Ser150 and water molecules. One of the calcium-ion sites has not been reported previously. This study further illustrates the involvement of small molecules in the crystallization of macromolecules through their ability to form intermolecular lattice interactions.
- Department of Molecular Biology and Biochemistry, The University of California, Irvine, 92697-3900, USA.
Organizational Affiliation: