NF-kapppaB p52:RelB heterodimer uses different binding modes to recognize different kappaB DNA
Fusco, A., Huang, D.B., Miller, D., Vu, D., Ghosh, G.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Avian reticuloendotheliosis viral (V-rel) oncogene related B | D [auth A] | 296 | Mus musculus | Mutation(s): 0 Gene Names: Relb | 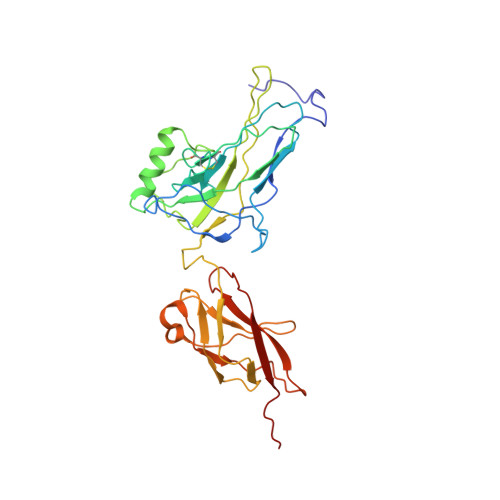 |
UniProt | |||||
Find proteins for Q04863 (Mus musculus) Explore Q04863 Go to UniProtKB: Q04863 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q04863 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Nuclear factor NF-kappa-B p100 subunit | E [auth B] | 293 | Homo sapiens | Mutation(s): 0 Gene Names: NFKB2, LYT10 | 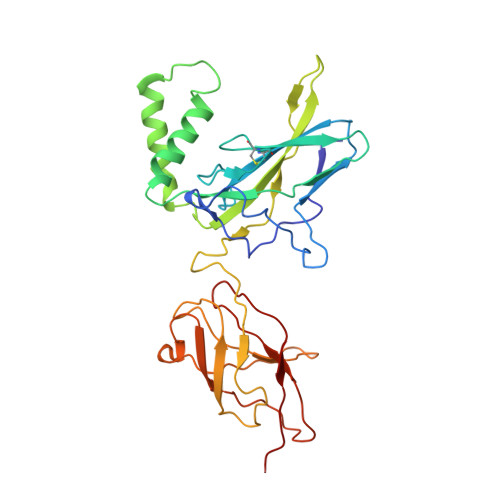 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q00653 (Homo sapiens) Explore Q00653 Go to UniProtKB: Q00653 | |||||
PHAROS: Q00653 GTEx: ENSG00000077150 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q00653 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar nucleic acids by: Sequence | 3D Structure
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | Organism | Image | |
| 5'-D(*DCP*DGP*DGP*DGP*DAP*DAP*DTP*DTP*DCP*DCP*DC)-3' | A [auth C], B [auth D], C [auth E] | 11 | N/A | 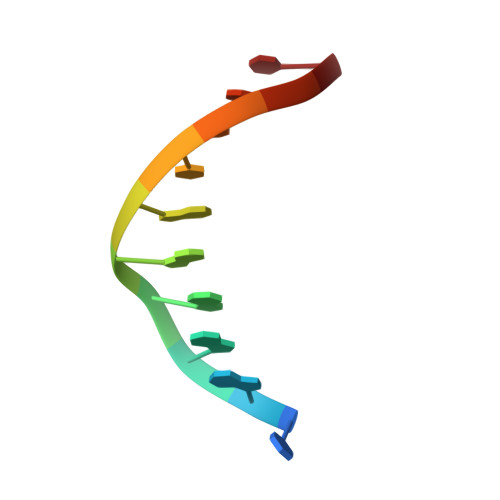 | |
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 77.64 | α = 90 |
| b = 124.13 | β = 90 |
| c = 189.59 | γ = 90 |
| Software Name | Purpose |
|---|---|
| CNS | refinement |
| HKL-2000 | data collection |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| AMoRE | phasing |