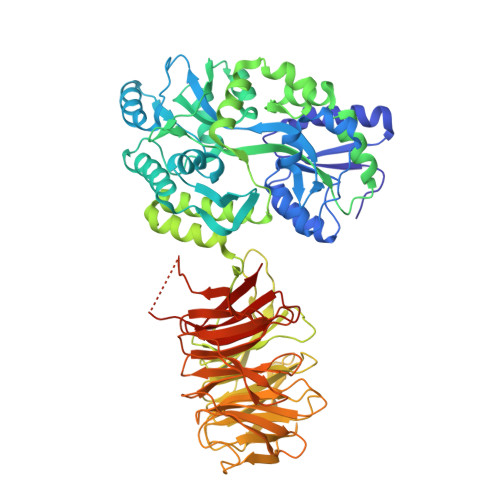
Structure of a signal transduction regulator, RACK1, from Arabidopsis thaliana.
Ullah, H., Scappini, E.L., Moon, A.F., Williams, L.V., Armstrong, D.L., Pedersen, L.C.(2008) Protein Sci 17: 1771-1780
- PubMed: 18715992
- DOI: https://doi.org/10.1110/ps.035121.108
- Primary Citation of Related Structures:
3DM0 - PubMed Abstract:
The receptor for activated C-kinase 1 (RACK1) is a highly conserved WD40 repeat scaffold protein found in a wide range of eukaryotic species from Chlamydymonas to plants and humans. In tissues of higher mammals, RACK1 is ubiquitously expressed and has been implicated in diverse signaling pathways involving neuropathology, cellular stress, protein translation, and developmental processes. RACK1 has established itself as a scaffold protein through physical interaction with a myriad of signaling proteins ranging from kinases, phosphatases, ion channels, membrane receptors, G proteins, IP3 receptor, and with widely conserved structural proteins associated with the ribosome. In the plant Arabidopsis thaliana, RACK1A is implicated in diverse developmental and environmental stress pathways. Despite the functional conservation of RACK1-mediated protein-protein interaction-regulated signaling modes, the structural basis of such interactions is largely unknown. Here we present the first crystal structure of a RACK1 protein, RACK1 isoform A from Arabidopsis thaliana, at 2.4 A resolution, as a C-terminal fusion of the maltose binding protein. The structure implicates highly conserved surface residues that could play critical roles in protein-protein interactions and reveals the surface location of proposed post-transcriptionally modified residues. The availability of this structure provides a structural basis for dissecting RACK1-mediated cellular signaling mechanisms in both plants and animals.
- Department of Biology, Howard University, Washington, DC 20059, USA.
Organizational Affiliation: