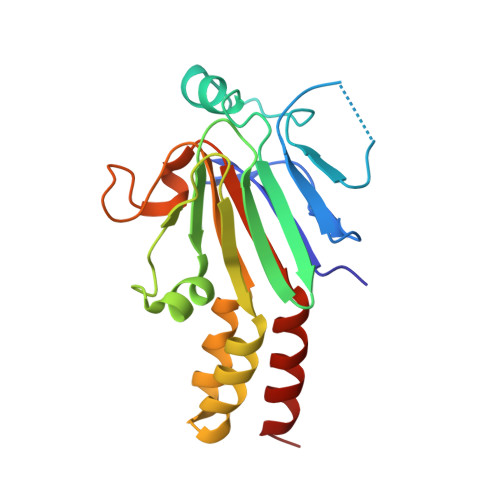
Structure of Drosophila Mad MH2 domain.
Hao, R., Chen, L., Wu, J.W., Wang, Z.X.(2008) Acta Crystallogr Sect F Struct Biol Cryst Commun 64: 986-990
- PubMed: 18997322
- DOI: https://doi.org/10.1107/S1744309108033034
- Primary Citation of Related Structures:
3DIT - PubMed Abstract:
In Drosophila, decapentaplegic (Dpp), a member of the TGF-beta superfamily, plays a pivotal role in control of proliferation, global patterning and induction of specific cell fates. Together with Medea, mother against Dpp (Mad), the founding member of the Smad family, specifically transduces the Dpp signal from the plasma membrane to the nucleus. Here, the crystal structure of the MH2 domain of Mad, which closely matches those of other Smad MH2 domains, is reported at 3.2 A resolution. The conservation of Smad protein structures is consistent with their evolutionary conserved and significant function. Furthermore, sequence alignment revealed that most of the variant amino acids in Smad proteins specific to the BMP pathway (Smad1, Smad5 and Mad) were clustered at the surface. In particular, Ser296 and Asp297 of Mad introduced a negative patch into the positive surface observed in the surface electrostatic potential of Smad1 MH2.
- Institute of Biophysics and Graduate University, Chinese Academy of Sciences, Beijing 100101, People's Republic of China.
Organizational Affiliation: