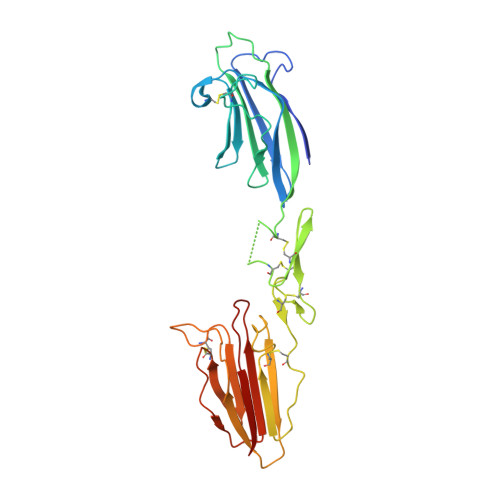
Crystal structure of the CUB1-EGF-CUB2 domain of human MASP-1/3 and identification of its interaction sites with mannan-binding lectin and ficolins
Teillet, F., Gaboriaud, C., Lacroix, M., Martin, L., Arlaud, G.J., Thielens, N.M.(2008) J Biological Chem 283: 25715-25724
- PubMed: 18596036
- DOI: https://doi.org/10.1074/jbc.M803551200
- Primary Citation of Related Structures:
3DEM - PubMed Abstract:
MASP-1 and MASP-3 are homologous proteases arising from alternative splicing of the MASP1/3 gene. They include an identical CUB(1)-EGF-CUB(2)-CCP(1)-CCP(2) module array prolonged by different serine protease domains at the C-terminal end. The x-ray structure of the CUB(1)-EGF-CUB(2) domain of human MASP-1/3, responsible for interaction of MASP-1 and -3 with their partner proteins mannan-binding lectin (MBL) and ficolins, was solved to a resolution of 2.3A(.) The structure shows a head-to-tail homodimer mainly stabilized by hydrophobic interactions between the CUB(1) module of one monomer and the epidermal growth factor (EGF) module of its counterpart. A Ca(2+) ion bound primarily to both EGF modules stabilizes the intra- and inter-monomer CUB(1)-EGF interfaces. Additional Ca(2+) ions are bound to each CUB(1) and CUB(2) module through six ligands contributed by Glu(49), Asp(57), Asp(102), and Ser(104) (CUB(1)) and their counterparts Glu(216), Asp(226), Asp(263), and Ser(265) (CUB(2)), plus one and two water molecules, respectively. To identify the residues involved in interaction of MASP-1 and -3 with MBL and L- and H-ficolins, 27 point mutants of human MASP-3 were generated, and their binding properties were analyzed using surface plasmon resonance spectroscopy. These mutations map two homologous binding sites contributed by modules CUB(1) and CUB(2), located in close vicinity of their Ca(2+)-binding sites and stabilized by the Ca(2+) ion. This information allows us to propose a model of the MBL-MASP-1/3 interaction, involving a major electrostatic interaction between two acidic Ca(2+) ligands of MASP-1/3 and a conserved lysine of MBL. Based on these and other data, a schematic model of a MBL.MASP complex is proposed.
- Laboratoire d'Enzymologie Moléculaire, 38027 Grenoble Cedex 1, France.
Organizational Affiliation: