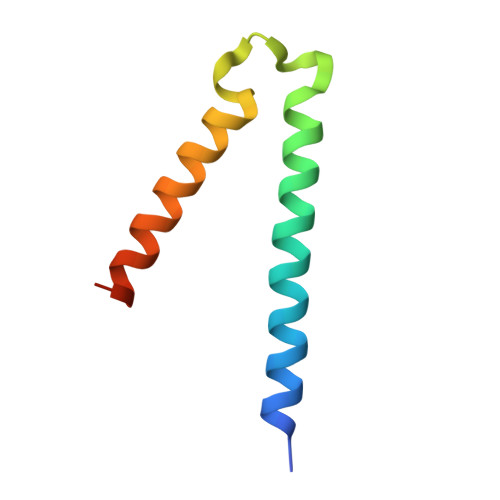
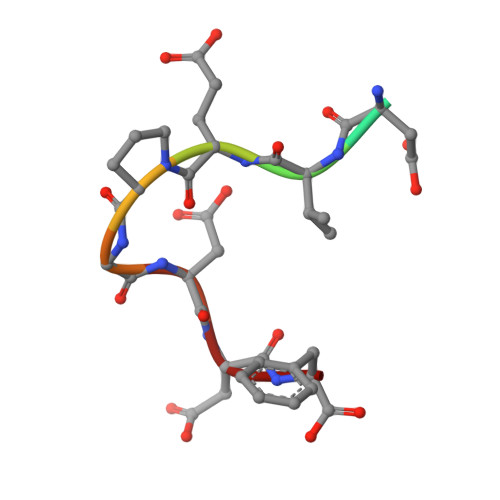
Structural basis of specific TraD-TraM recognition during F plasmid-mediated bacterial conjugation.
Lu, J., Wong, J.J., Edwards, R.A., Manchak, J., Frost, L.S., Glover, J.N.(2008) Mol Microbiol 70: 89-99
- PubMed: 18717787
- DOI: https://doi.org/10.1111/j.1365-2958.2008.06391.x
- Primary Citation of Related Structures:
3D8A - PubMed Abstract:
F plasmid-mediated bacterial conjugation requires interactions between a relaxosome component, TraM, and the coupling protein TraD, a hexameric ring ATPase that forms the cytoplasmic face of the conjugative pore. Here we present the crystal structure of the C-terminal tail of TraD bound to the TraM tetramerization domain, the first structural evidence of relaxosome-coupling protein interactions. The structure reveals the TraD C-terminal peptide bound to each of four symmetry-related grooves on the surface of the TraM tetramer. Extensive protein-protein interactions were observed between the two proteins. Mutational analysis indicates that these interactions are specific and required for efficient F conjugation in vivo. Our results suggest that specific interactions between the C-terminal tail of TraD and the TraM tetramerization domain might lead to more generalized interactions that stabilize the relaxosome-coupling protein complex in preparation for conjugative DNA transfer.
- Departments of Biochemistry, University of Alberta, Edmonton, Alberta, Canada.
Organizational Affiliation: