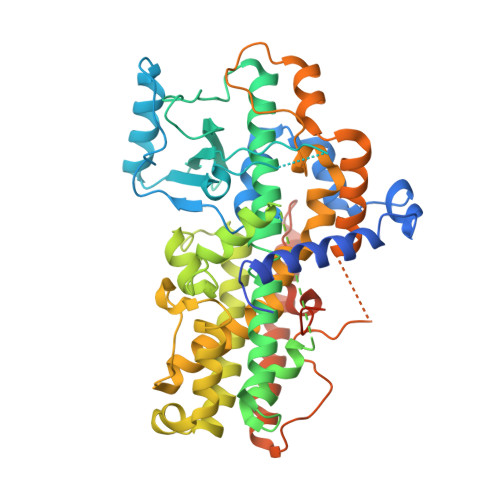
Crystal structure of the lipoxygenase domain of human Arachidonate 12-lipoxygenase, 12S-type.
Tresaugues, L., Moche, M., Arrowsmith, C.H., Berglund, H., Busam, R.D., Collins, R., Dahlgren, L.G., Edwards, A.M., Flodin, S., Flores, A., Graslund, S., Hammarstrom, M., Herman, M.D., Johansson, A., Johansson, I., Kallas, A., Karlberg, T., Kotenyova, T., Lehtio, L., Nilsson, M.E., Nyman, T., Olesen, K., Persson, C., Sagemark, J., Schueler, H., Svensson, L., Thorsell, A.G., Van Den Berg, S., Welin, M., Weigelt, J., Wikstrom, M., Nordlund, P.To be published.