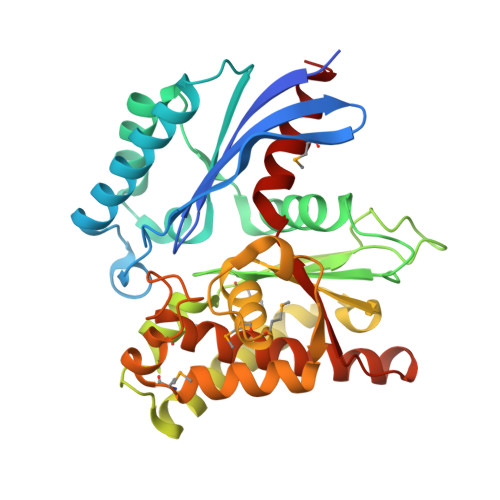
Crystal structure of the exopolyphosphatase-like protein Q8G5J2. Northeast Structural Genomics Consortium target BlR13.
Kuzin, A.P., Su, M., Yang, C., Neely, H., Seetharaman, J., Shastry, R., Fang, Y., Cunningham, K., Ma, L.-C., Xiao, R., Liu, J., Baran, M.C., Acton, T.B., Rost, B., Montelione, G.T., Hunt, J.F., Tong, L.To be published.