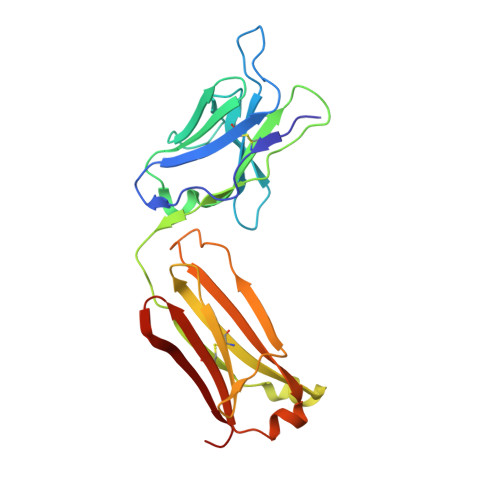
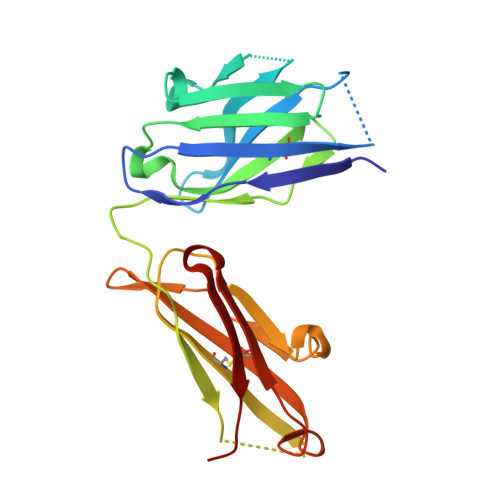
Structures of synthetic O-antigen fragments from serotype 2a Shigella flexneri in complex with a protective monoclonal antibody
Vulliez-Le Normand, B., Saul, F.A., Phalipon, A., Belot, F., Guerreiro, C., Mulard, L.A., Bentley, G.A.(2008) Proc Natl Acad Sci U S A 105: 9976-9981
- PubMed: 18621718
- DOI: https://doi.org/10.1073/pnas.0801711105
- Primary Citation of Related Structures:
3BZ4, 3C5S, 3C6S - PubMed Abstract:
The anti-LPS IgG mAb F22-4, raised against Shigella flexneri serotype 2a bacteria, protects against homologous, but not heterologous, challenge in an experimental animal model. We report the crystal structures of complexes formed between Fab F22-4 and two synthetic oligosaccharides, a decasaccharide and a pentadecasaccharide that were previously shown to be both immunogenic and antigenic mimics of the S. flexneri serotype 2a O-antigen. F22-4 binds to an epitope contained within two consecutive 2a serotype pentasaccharide repeat units (RU). Six sugar residues from a contiguous nine-residue segment make direct contacts with the antibody, including the nonreducing rhamnose and both branching glucosyl residues from the two RUs. The glucosyl residue, whose position of attachment to the tetrasaccharide backbone of the RU defines the serotype 2a O-antigen, is critical for recognition by F22-4. Although the complete decasaccharide is visible in the electron density maps, the last four pentadecasaccharide residues from the reducing end, which do not contact the antibody, could not be traced. Although considerable mobility in the free oligosaccharides can thus be expected, the conformational similarity between the individual RUs, both within and between the two complexes, suggests that short-range transient ordering to a helical conformation might occur in solution. Although the observed epitope includes the terminal nonreducing residue, binding to internal epitopes within the polysaccharide chain is not precluded. Our results have implications for vaccine development because they suggest that a minimum of two RUs of synthetic serotype 2a oligosaccharide is required for optimal mimicry of O-Ag epitopes.
- Institut Pasteur, Unité d'Immunologie Structurale, Centre National de la Recherche Scientifique Unité de Recherche Associée 2185, F-75015 Paris, France. bvulliez@pasteur.fr
Organizational Affiliation: