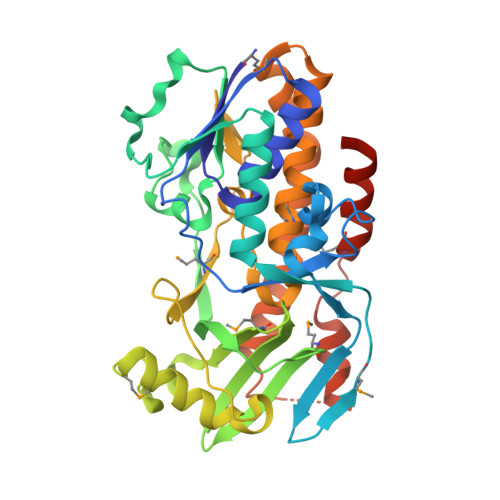
Crystal structure of vioD hydroxylase in complex with FAD from Chromobacterium violaceum.
Forouhar, F., Neely, H., Seetharaman, J., Janjua, H., Xiao, R., Maglaqui, M., Wang, H., Baran, M.C., Acton, T.B., Montelione, G.T., Hunt, J.F., Tong, L.To be published.