Conformational Transitions Revealed by Structures of Acylphosphatase from Bacillus subtilis in Different States
Hu, J.C., Li, D., Su, X.D., Jin, C.W., Xia, B.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Probable acylphosphatase | 91 | Bacillus subtilis | Mutation(s): 0 Gene Names: yflL EC: 3.6.1.7 | 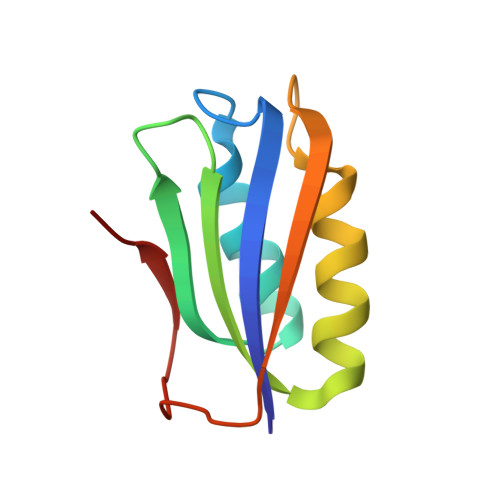 | |
UniProt | |||||
Find proteins for O35031 (Bacillus subtilis (strain 168)) Explore O35031 Go to UniProtKB: O35031 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O35031 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| PO4 Query on PO4 | B [auth A] | PHOSPHATE ION O4 P NBIIXXVUZAFLBC-UHFFFAOYSA-K |  | ||
| GOL Query on GOL | C [auth A] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 26.857 | α = 90 |
| b = 48.27 | β = 90 |
| c = 59.567 | γ = 90 |
| Software Name | Purpose |
|---|---|
| DENZO | data reduction |
| SCALEPACK | data scaling |
| CNS | refinement |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| HKL-2000 | data collection |
| CNS | phasing |