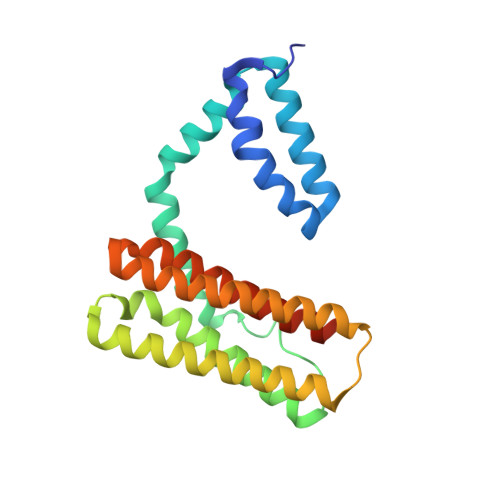
Crystal structure of human programmed cell death 10 complexed with inositol-(1,3,4,5)-tetrakisphosphate: a novel adaptor protein involved in human cerebral cavernous malformation.
Ding, J., Wang, X., Li, D.F., Hu, Y., Zhang, Y., Wang, D.C.(2010) Biochem Biophys Res Commun 399: 587-592
- PubMed: 20682288
- DOI: https://doi.org/10.1016/j.bbrc.2010.07.119
- Primary Citation of Related Structures:
3AJM - PubMed Abstract:
Programmed cell death 10 (PDCD10) is a novel adaptor protein involved in human cerebral cavernous malformation, a common vascular lesion mostly occurring in the central nervous system. By interacting with different signal proteins, PDCD10 could regulate various physiological processes in the cell. The crystal structure of human PDCD10 complexed with inositol-(1,3,4,5)-tetrakisphosphate has been determined at 2.3A resolution. The structure reveals an integrated dimer via a unique assembly that has never been observed before. Each PDCD10 monomer contains two independent domains: an N-terminal domain with a new fold involved in the tight dimer assembly and a C-terminal four-helix bundle domain that closely resembles the focal adhesion targeting domain of focal adhesion kinase. An eight-residue flexible linker connects the two domains, potentially conferring mobility onto the C-terminal domain, resulting in the conformational variability of PDCD10. A variable basic cleft on the top of the dimer interface binds to phosphatidylinositide and regulates the intracellular localization of PDCD10. Two potential sites, respectively located on the two domains, are critical for recruiting different binding partners, such as germinal center kinase III proteins and the focal adhesion protein paxillin.
- National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, People's Republic of China.
Organizational Affiliation: