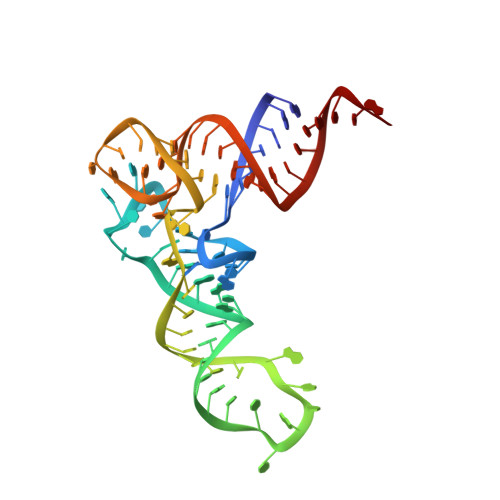
Structural basis for translational fidelity ensured by transfer RNA lysidine synthetase.
Nakanishi, K., Bonnefond, L., Kimura, S., Suzuki, T., Ishitani, R., Nureki, O.(2009) Nature 461: 1144-1148
- PubMed: 19847269
- DOI: https://doi.org/10.1038/nature08474
- Primary Citation of Related Structures:
3A2K, 3HJ7 - PubMed Abstract:
Maturation of precursor transfer RNA (pre-tRNA) includes excision of the 5' leader and 3' trailer sequences, removal of introns and addition of the CCA terminus. Nucleotide modifications are incorporated at different stages of tRNA processing, after the RNA molecule adopts the proper conformation. In bacteria, tRNA(Ile2) lysidine synthetase (TilS) modifies cytidine into lysidine (L; 2-lysyl-cytidine) at the first anticodon of tRNA(Ile2) (refs 4-9). This modification switches tRNA(Ile2) from a methionine-specific to an isoleucine-specific tRNA. However, the aminoacylation of tRNA(Ile2) by methionyl-tRNA synthetase (MetRS), before the modification by TilS, might lead to the misincorporation of methionine in response to isoleucine codons. The mechanism used by bacteria to avoid this pitfall is unknown. Here we show that the TilS enzyme specifically recognizes and modifies tRNA(Ile2) in its precursor form, thereby avoiding translation errors. We identified the lysidine modification in pre-tRNA(Ile2) isolated from RNase-E-deficient Escherichia coli and did not detect mature tRNA(Ile2) lacking this modification. Our kinetic analyses revealed that TilS can modify both types of RNA molecule with comparable efficiencies. X-ray crystallography and mutational analyses revealed that TilS specifically recognizes the entire L-shape structure in pre-tRNA(Ile2) through extensive interactions coupled with sequential domain movements. Our results demonstrate how TilS prevents the recognition of tRNA(Ile2) by MetRS and achieves high specificity for its substrate. These two key points form the basis for maintaining the fidelity of isoleucine codon translation in bacteria. Our findings also provide a rationale for the necessity of incorporating specific modifications at the precursor level during tRNA biogenesis.
- Department of Biological Information, Graduate School of Bioscience and Biotechnology, Tokyo Institute of Technology, Yokohama, Kanagawa 225-8501, Japan.
Organizational Affiliation: