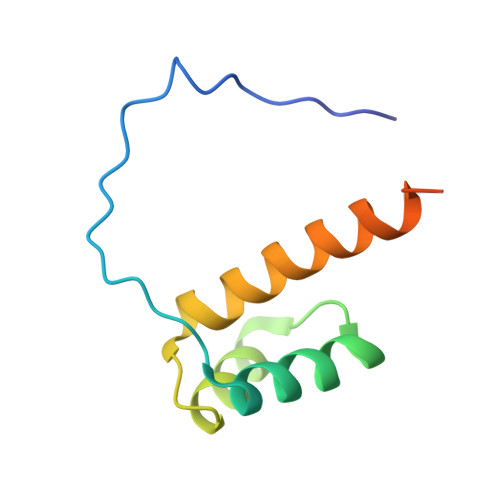
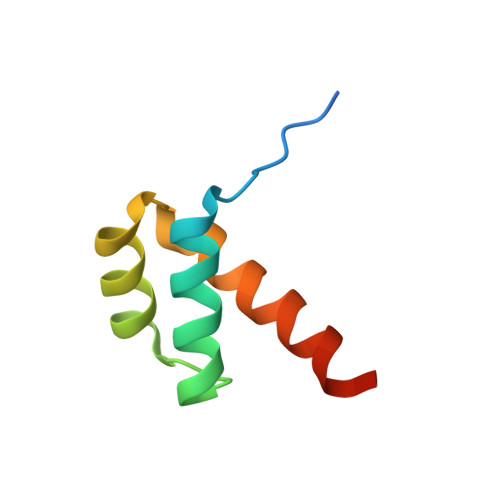
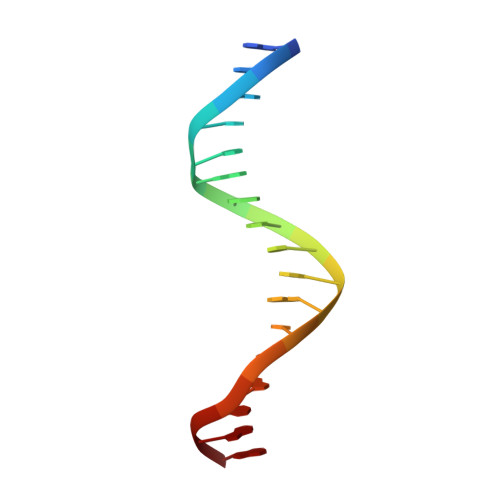
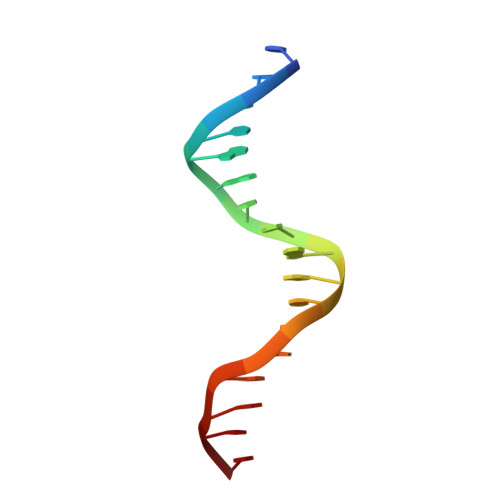
Cooperative DNA-binding and sequence-recognition mechanism of aristaless and clawless
Miyazono, K., Zhi, Y., Takamura, Y., Nagata, K., Saigo, K., Kojima, T., Tanokura, M.(2010) EMBO J 29: 1613-1623
- PubMed: 20389279
- DOI: https://doi.org/10.1038/emboj.2010.53
- Primary Citation of Related Structures:
3A01, 3A02, 3A03, 3LNQ - PubMed Abstract:
To achieve accurate gene regulation, some homeodomain proteins bind cooperatively to DNA to increase those site specificities. We report a ternary complex structure containing two homeodomain proteins, aristaless (Al) and clawless (Cll), bound to DNA. Our results show that the extended conserved sequences of the Cll homeodomain are indispensable to cooperative DNA binding. In the Al-Cll-DNA complex structure, the residues in the extended regions are used not only for the intermolecular contacts between the two homeodomain proteins but also for the sequence-recognition mechanism of DNA by direct interactions. The residues in the extended N-terminal arm lie within the minor groove of DNA to form direct interactions with bases, whereas the extended conserved region of the C-terminus of the homeodomain interacts with Al to stabilize and localize the third alpha helix of the Cll homeodomain. This structure suggests a novel mode for the cooperativity of homeodomain proteins.
- Department of Applied Biological Chemistry, University of Tokyo, Bunkyo-ku, Tokyo, Japan.
Organizational Affiliation: