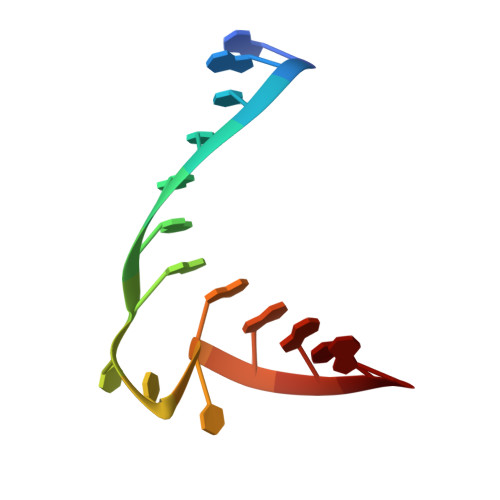
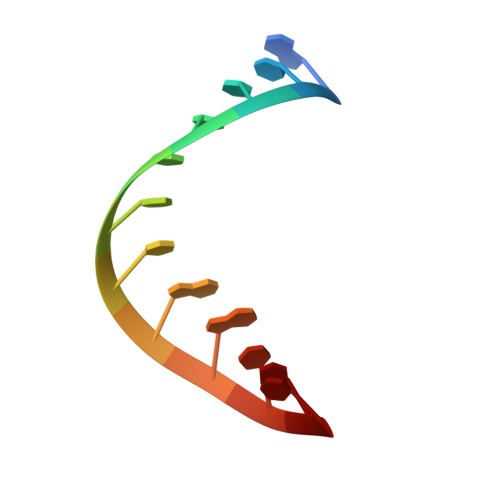
A 1.3-A resolution crystal structure of the HIV-1 trans-activation response region RNA stem reveals a metal ion-dependent bulge conformation.
Ippolito, J.A., Steitz, T.A.(1998) Proc Natl Acad Sci U S A 95: 9819-9824
- PubMed: 9707559
- DOI: https://doi.org/10.1073/pnas.95.17.9819
- Primary Citation of Related Structures:
397D - PubMed Abstract:
The crystal structure of an HIV-1 trans-activation response region (TAR) RNA fragment containing the binding site for the trans-activation protein Tat has been determined to 1.3-A resolution. In this crystal structure, the characteristic UCU bulge of TAR adopts a conformation that is stabilized by three divalent calcium ions and differs from those determined previously by solution NMR. One metal ion, crucial to the loop conformation, binds directly to three phosphates in the loop region. The structure emphasizes the influence of metal ion binding on RNA structure and, given the abundance of divalent metal ion in the cell, raises the question of whether metal ions play a role in the conformation of TAR RNA and the interaction of TAR with Tat and cyclin T in vivo.
- Department of Molecular Biophysics and Biochemistry,, New Haven, CT 06520-8114, USA.
Organizational Affiliation: