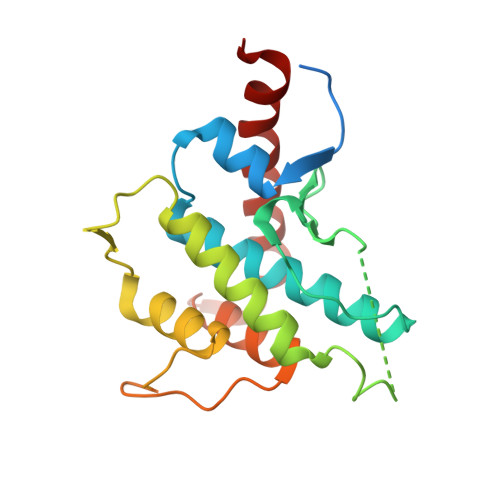
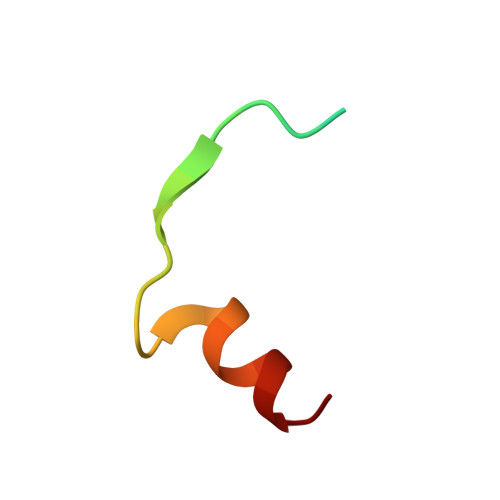
Molecular Basis of Actin Nucleation Factor Cooperativity: Crystal Structure of the Spir-1 Kinase Non-Catalytic C-Lobe Domain (Kind)Formin-2 Formin Spir Interaction Motif (Fsi) Complex.
Zeth, K., Pechlivanis, M., Samol, A., Pleiser, S., Vonrhein, C., Kerkhoff, E.(2011) J Biological Chem 286: 30732
- PubMed: 21705804
- DOI: https://doi.org/10.1074/jbc.M111.257782
- Primary Citation of Related Structures:
2YLE, 2YLF - PubMed Abstract:
The distinct actin nucleation factors of the Spir and formin subgroup families cooperate in actin nucleation. The Spir/formin cooperativity has been identified to direct two essential steps in mammalian oocyte maturation, the asymmetric spindle positioning and polar body extrusion during meiosis. Understanding the nature and regulation of the Spir/Fmn cooperation is an important requirement to comprehend mammalian reproduction. Recently we dissected the structural elements of the Spir and Fmn family proteins, which physically link the two actin nucleation factors. The trans-regulatory interaction is mediated by the Spir kinase non-catalytic C-lobe domain (KIND) and the C-terminal formin Spir interaction motif (FSI). The interaction inhibits formin nucleation activity and enhances the Spir activity. To get insights into the molecular mechanism of the Spir/Fmn interaction, we determined the crystal structure of the KIND domain alone and in complex with the C-terminal Fmn-2 FSI peptide. Together they confirm the proposed structural homology of the KIND domain to the protein kinase fold and reveal the basis of the Spir/formin interaction. The complex structure showed a large interface with conserved and positively charged residues of the Fmn FSI peptide mediating major contacts to an acidic groove on the surface of KIND. Protein interaction studies verified the electrostatic nature of the interaction. The data presented here provide the molecular basis of the Spir/formin interaction and give a first structural view into the mechanisms of actin nucleation factor cooperativity.
- Department of Protein Evolution, Max Planck Institute for Developmental Biology, Spemannstrasse 35, 72076 Tübingen, Germany.
Organizational Affiliation: