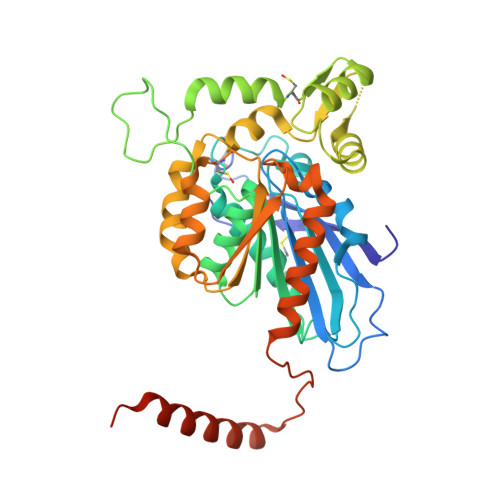
The Unusual Extended C-Terminal Helix of the Peroxisomal Alpha-Beta-Hydrolase Lpx1 is Involved in Dimer Contacts But Dispensable for Dimerization
Thoms, S., Hofhuis, J., Thoing, C., Gartner, J., Niemann, H.H.(2011) J Struct Biol 175: 362
- PubMed: 21741480
- DOI: https://doi.org/10.1016/j.jsb.2011.06.008
- Primary Citation of Related Structures:
2Y6U, 2Y6V - PubMed Abstract:
The yeast peroxisomal hydrolase Lpx1 belongs to the α/β-hydrolase superfamily. In the absence of Lpx1, yeast peroxisomes show an aberrant vacuolated morphology similar to what is found in peroxisomal disorder patients. Here, we present the crystal structure of Lpx1 determined at a resolution of 1.9 Å. The structure reveals the complete catalytic triad with an unusual location of the acid residue after strand β6 of the canonical α/β-hydrolase fold. A four-helix cap domain covers the active site. The interface between the α/β-hydrolase core and the cap domain forms the potential substrate binding site, which may also comprise the tunnel that leads into the protein interior and widens into a cavity. Two further tunnels connect the active site to the protein surface, potentially facilitating substrate access. Lpx1 is a homodimer. The α/β-hydrolase core folds of the two protomers form the dimer contact site. Further dimerization contacts arise from the mutual embracement of the cap domain of one protomer by the non-canonical C-terminal helix of the other, resulting in a total buried surface area of some 6000 Ų. The unusual C-terminal helix sticks out from the core fold to which it is connected by an extended flexible loop. We analyzed whether this helix is required for dimerization and for import of the dimer into peroxisomes using biochemical assays in vitro and a microscopy-based interaction assay in mammalian cells. Surprisingly, the C-terminal helix is dispensable for dimerization and dimer import. The unusually robust self-interaction suggests that Lpx1 is imported into peroxisomes as dimer.
- Department of Pediatrics and Pediatric Neurology, University Medical Center, University of Göttingen, Robert-Koch-Str. 40, 37075 Göttingen, Germany. sven.thoms@med.uni-goettingen.de
Organizational Affiliation: