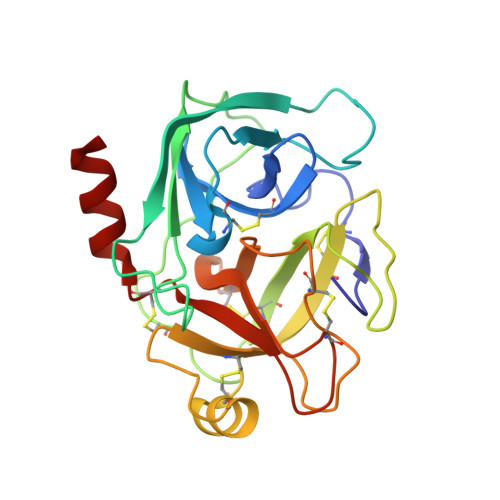
The catalytic aspartate is protonated in the Michaelis complex formed between trypsin and an in vitro evolved substrate-like inhibitor: a refined mechanism of serine protease action.
Wahlgren, W.Y., Pal, G., Kardos, J., Porrogi, P., Szenthe, B., Patthy, A., Graf, L., Katona, G.(2011) J Biological Chem 286: 3587-3596
- PubMed: 21097875
- DOI: https://doi.org/10.1074/jbc.M110.161604
- Primary Citation of Related Structures:
2XTT - PubMed Abstract:
The mechanism of serine proteases prominently illustrates how charged amino acid residues and proton transfer events facilitate enzyme catalysis. Here we present an ultrahigh resolution (0.93 Å) x-ray structure of a complex formed between trypsin and a canonical inhibitor acting through a substrate-like mechanism. The electron density indicates the protonation state of all catalytic residues where the catalytic histidine is, as expected, in its neutral state prior to the acylation step by the catalytic serine. The carboxyl group of the catalytic aspartate displays an asymmetric electron density so that the O(δ2)-C(γ) bond appears to be a double bond, with O(δ2) involved in a hydrogen bond to His-57 and Ser-214. Only when Asp-102 is protonated on O(δ1) atom could a density functional theory simulation reproduce the observed electron density. The presence of a putative hydrogen atom is also confirmed by a residual mF(obs) - DF(calc) density above 2.5 σ next to O(δ1). As a possible functional role for the neutral aspartate in the active site, we propose that in the substrate-bound form, the neutral aspartate residue helps to keep the pK(a) of the histidine sufficiently low, in the active neutral form. When the histidine receives a proton during the catalytic cycle, the aspartate becomes simultaneously negatively charged, providing additional stabilization for the protonated histidine and indirectly to the tetrahedral intermediate. This novel proposal unifies the seemingly conflicting experimental observations, which were previously seen as either supporting the charge relay mechanism or the neutral pK(a) histidine theory.
- Department of Chemistry, University of Gothenburg, Medicinaregatan 9C, 40530 Gothenburg, Sweden.
Organizational Affiliation: