An Ion-Channel Modulator from the Saliva of the Brown Ear Tick Has a Highly Modified Kunitz/Bpti Structure.
Paesen, G.C., Siebold, C., Dallas, M.L., Peers, C., Harlos, K., Nuttall, P.A., Nunn, M.A., Stuart, D.I., Esnouf, R.M.(2009) J Mol Biology 389: 734
- PubMed: 19394347
- DOI: https://doi.org/10.1016/j.jmb.2009.04.045
- Primary Citation of Related Structures:
2W8X - PubMed Abstract:
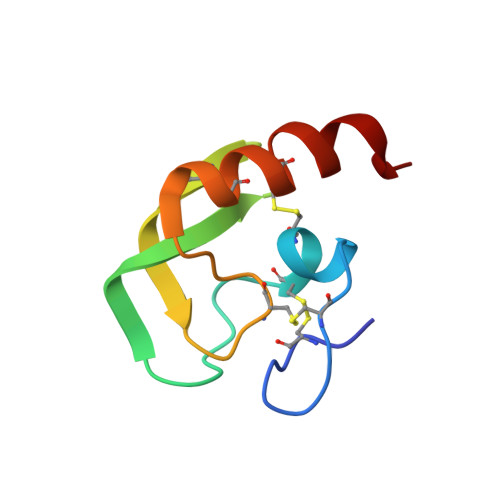
Ra-KLP, a 75 amino acid protein secreted by the salivary gland of the brown ear tick Rhipicephalus appendiculatus has a sequence resembling those of Kunitz/BPTI proteins. We report the detection, purification and characterization of the function of Ra-KLP. In addition, determination of the three-dimensional crystal structure of Ra-KLP at 1.6 A resolution using sulphur single-wavelength anomalous dispersion reveals that much of the loop structure of classical Kunitz domains, including the protruding protease-binding loop, has been replaced by beta-strands. Even more unusually, the N-terminal portion of the polypeptide chain is pinned to the "Kunitz head" by two disulphide bridges not found in classical Kunitz/BPTI proteins. The disulphide bond pattern has been further altered by the loss of the bridge that normally stabilizes the protease-binding loop. Consistent with the conversion of this loop into a beta-strand, Ra-KLP shows no significant anti-protease activity; however, it activates maxiK channels in an in vitro system, suggesting a potential mechanism for regulating host blood supply during feeding.
- CEH Oxford, Mansfield Road, Oxford OX1 3SR, UK. gcp@ceh.ac.uk
Organizational Affiliation: