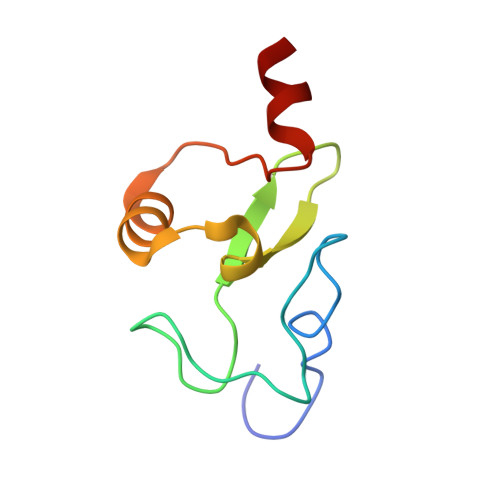
The Plant Homeodomain Finger of Rag2 Recognizes Histone H3 Methylated at Both Lysine-4 and Arginine-2.
Ramon-Maiques, S., Kuo, A.J., Carney, D., Matthews, A.G.W., Oettinger, M.A., Gozani, O., Yang, W.(2007) Proc Natl Acad Sci U S A 104: 18993
- PubMed: 18025461
- DOI: https://doi.org/10.1073/pnas.0709170104
- Primary Citation of Related Structures:
2V83, 2V85, 2V86, 2V87, 2V88 - PubMed Abstract:
Recombination activating gene (RAG) 1 and RAG2 together catalyze V(D)J gene rearrangement in lymphocytes as the first step in the assembly and maturation of antigen receptors. RAG2 contains a plant homeodomain (PHD) near its C terminus (RAG2-PHD) that recognizes histone H3 methylated at lysine 4 (H3K4me) and influences V(D)J recombination. We report here crystal structures of RAG2-PHD alone and complexed with five modified H3 peptides. Two aspects of RAG2-PHD are unique. First, in the absence of the modified peptide, a peptide N-terminal to RAG2-PHD occupies the substrate-binding site, which may reflect an autoregulatory mechanism. Second, in contrast to other H3K4me3-binding PHD domains, RAG2-PHD substitutes a carboxylate that interacts with arginine 2 (R2) with a Tyr, resulting in binding to H3K4me3 that is enhanced rather than inhibited by dimethylation of R2. Five residues involved in histone H3 recognition were found mutated in severe combined immunodeficiency (SCID) patients. Disruption of the RAG2-PHD structure appears to lead to the absence of T and B lymphocytes, whereas failure to bind H3K4me3 is linked to Omenn Syndrome. This work provides a molecular basis for chromatin-dependent gene recombination and presents a single protein domain that simultaneously recognizes two distinct histone modifications, revealing added complexity in the read-out of combinatorial histone modifications.
- Laboratory of Molecular Biology, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892, USA.
Organizational Affiliation: