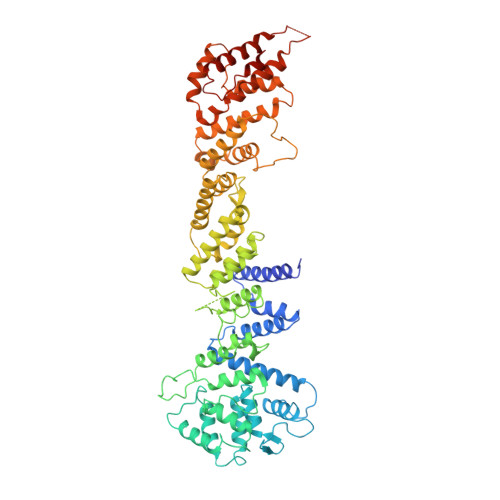
Structural basis of the nic96 subcomplex organization in the nuclear pore channel.
Schrader, N., Stelter, P., Flemming, D., Kunze, R., Hurt, E., Vetter, I.R.(2008) Mol Cell 29: 46-55
- PubMed: 18206968
- DOI: https://doi.org/10.1016/j.molcel.2007.10.022
- Primary Citation of Related Structures:
2RFO - PubMed Abstract:
Nic96 is a conserved nucleoporin that recruits the Nsp1-Nup49-Nup57 complex, a module with Phe-Gly (FG) repeats, to the central transport channel of the nuclear pore complex (NPC). Nic96 binds the Nsp1 complex via its N domain and assembles into the NPC framework via its central and C domain. Here, we report the crystal structure of a large structural nucleoporin, Nic96 without its N domain (Nic96DeltaN). Nic96DeltaN is composed of three domains and is a straight molecule that--although almost entirely helical--exhibits strong deviations from the predicted alpha-solenoid fold. The missing N domain projects midway from the Nic96 molecule, indicating how the Nsp1 complex might be located with respect to the rod-like Nic96. Notably, Nic96DeltaN binds in vitro to FG repeats of the Nsp1 complex. These data suggest a model of how Nic96 could organize a transport module with coiled-coil domains and FG repeats in the central pore channel.
- Max-Planck-Institut für Molekulare Physiologie, Abteilung Strukturelle Biologie, Otto-Hahn-Strasse 11, 44227 Dortmund, Germany.
Organizational Affiliation: