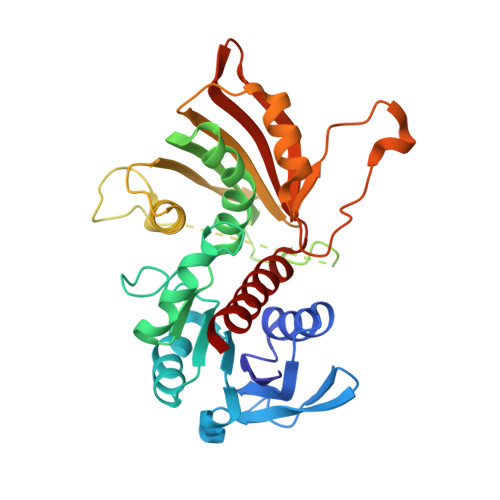
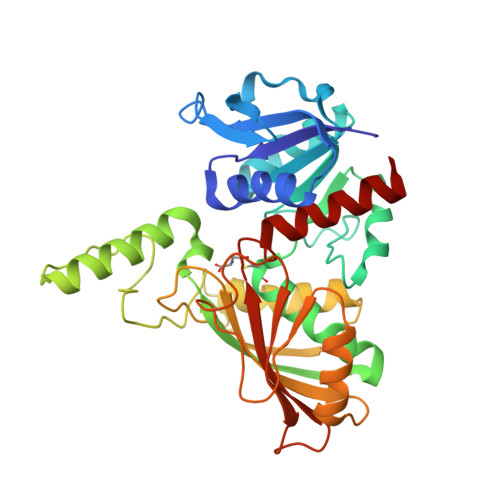
The structure of a redundant enzyme: a second isoform of aspartate beta-semialdehyde dehydrogenase in Vibrio cholerae.
Viola, R.E., Liu, X., Ohren, J.F., Faehnle, C.R.(2008) Acta Crystallogr D Biol Crystallogr 64: 321-330
- PubMed: 18323627
- DOI: https://doi.org/10.1107/S0907444907068552
- Primary Citation of Related Structures:
2QZ9, 2R00 - PubMed Abstract:
Aspartate-beta-semialdehyde dehydrogenase (ASADH) is an essential enzyme that is found in bacteria, fungi and plants but not in humans. ASADH produces the first branch-point metabolite in the biosynthetic pathways that lead to the production of lysine, threonine, methionine and isoleucine as well as the cell-wall precursor diaminopimelate. As a consequence, ASADH appears to be an excellent target for the development of novel antibiotics, especially for Gram-negative bacteria that require diaminopimelate for cell-wall biosynthesis. In contrast to the Gram-negative ASADHs, which readily formed well diffracting crystals, the second isoform of aspartate-beta-semialdehyde dehydrogenase from Vibrio cholerae (vcASADH2) was less well behaved in initial crystallization trials. In order to obtain good-quality single crystals of vcASADH2, a buffer-optimization protocol was used in which the initial purification buffer was exchanged into a new condition derived from a pre-crystalline hit. The unliganded structure of vcASADH2 has been determined to 2.2 A resolution to provide additional insight into the structural and functional evolution of the ASADH enzyme family. The overall fold and domain organization of this new structure is similar to the Gram-negative, Gram-positive and archeal ASADH structures determined previously, despite having less than 50% sequence identity to any of these family members. The substrate-complex structure reveals that the binding of L-aspartate-beta-semialdehyde (ASA) to vcASADH2 is accommodated by structural changes in the amino-acid binding site and in the helical subdomain that is involved in the dimer interface. Structural alignments show that this second isoform from Gram-negative V. cholerae most closely resembles the ASADH from a Gram-positive organism and is likely to bind the coenzyme in a different conformation to that observed in the other V. cholerae isoform.
- Department of Chemistry, The University of Toledo, Toledo, Ohio 43606, USA. ron.viola@utoledo.edu
Organizational Affiliation: