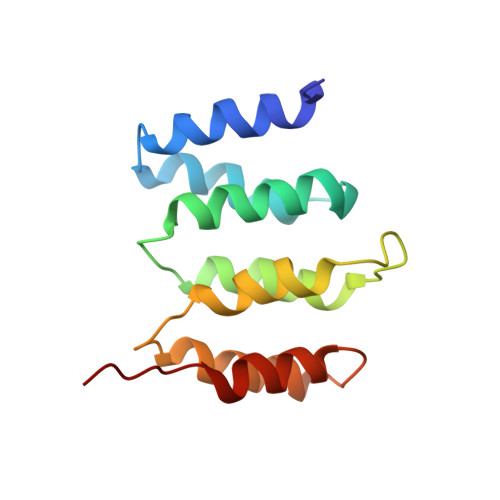
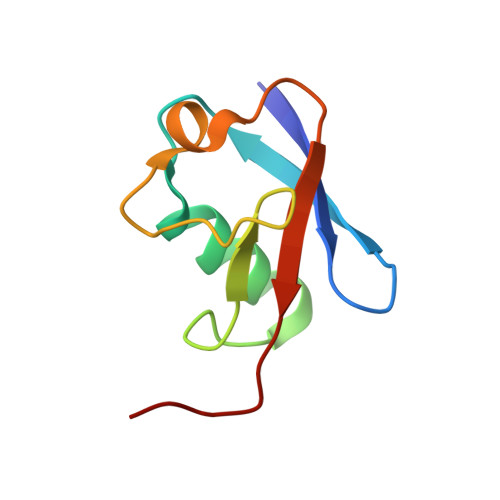
Structures of Rpn1 T1:Rad23 and hRpn13:hPLIC2 Reveal Distinct Binding Mechanisms between Substrate Receptors and Shuttle Factors of the Proteasome.
Chen, X., Randles, L., Shi, K., Tarasov, S.G., Aihara, H., Walters, K.J.(2016) Structure 24: 1257-1270
- PubMed: 27396824
- DOI: https://doi.org/10.1016/j.str.2016.05.018
- Primary Citation of Related Structures:
2NBU, 2NBV, 2NBW, 5IRS - PubMed Abstract:
Three receptors (Rpn1/S2/PSMD2, Rpn10/S5a, Rpn13/Adrm1) in the proteasome bind substrates by interacting with conjugated ubiquitin chains and/or shuttle factors (Rad23/HR23, Dsk2/PLIC/ubiquilin, Ddi1) that carry ubiquitinated substrates to proteasomes. We solved the structure of two such receptors with their preferred shuttle factor, namely hRpn13(Pru):hPLIC2(UBL) and scRpn1 T1:scRad23(UBL). We find that ubiquitin folds in Rad23 and Dsk2 are fine-tuned by residue substitutions to achieve high affinity for Rpn1 and Rpn13, respectively. A single substitution in hPLIC2 yields enhanced interactions with the Rpn13 ubiquitin contact surface and sterically blocks hRpn13 binding to its preferred ubiquitin chain type, K48-linked chains. Rpn1 T1 binds two ubiquitins in tandem and we find that Rad23 binds exclusively to the higher-affinity Helix28/Helix30 site. Rad23 contacts at Helix28/Helix30 are optimized compared to ubiquitin by multiple conservative amino acid substitutions. Thus, shuttle factors deliver substrates to proteasomes through fine-tuned ubiquitin-like surfaces.
- Protein Processing Section, Structural Biophysics Laboratory, Center for Cancer Research, National Cancer Institute, Frederick, MD 21702, USA.
Organizational Affiliation: