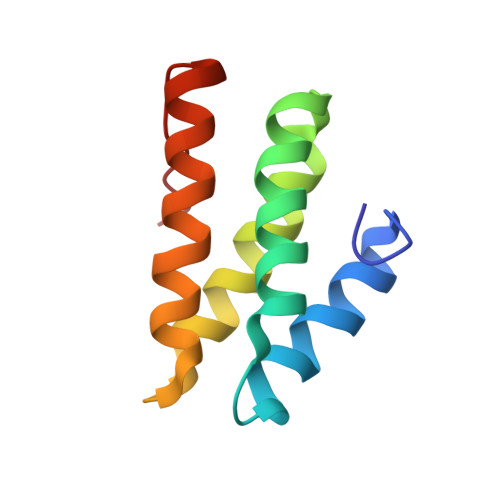
Interaction between the Rev1 C-Terminal Domain and the PolD3 Subunit of Pol zeta Suggests a Mechanism of Polymerase Exchange upon Rev1/Pol zeta-Dependent Translesion Synthesis.
Pustovalova, Y., Magalhaes, M.T., D'Souza, S., Rizzo, A.A., Korza, G., Walker, G.C., Korzhnev, D.M.(2016) Biochemistry 55: 2043-2053
- PubMed: 26982350
- DOI: https://doi.org/10.1021/acs.biochem.5b01282
- Primary Citation of Related Structures:
2N1G - PubMed Abstract:
Translesion synthesis (TLS) is a mutagenic branch of cellular DNA damage tolerance that enables bypass replication over DNA lesions carried out by specialized low-fidelity DNA polymerases. The replicative bypass of most types of DNA damage is performed in a two-step process of Rev1/Polζ-dependent TLS. In the first step, a Y-family TLS enzyme, typically Polη, Polι, or Polκ, inserts a nucleotide across a DNA lesion. In the second step, a four-subunit B-family DNA polymerase Polζ (Rev3/Rev7/PolD2/PolD3 complex) extends the distorted DNA primer-template. The coordinated action of error-prone TLS enzymes is regulated through their interactions with the two scaffold proteins, the sliding clamp PCNA and the TLS polymerase Rev1. Rev1 interactions with all other TLS enzymes are mediated by its C-terminal domain (Rev1-CT), which can simultaneously bind the Rev7 subunit of Polζ and Rev1-interacting regions (RIRs) from Polη, Polι, or Polκ. In this work, we identified a previously unknown RIR motif in the C-terminal part of PolD3 subunit of Polζ whose interaction with the Rev1-CT is among the tightest mediated by RIR motifs. Three-dimensional structure of the Rev1-CT/PolD3-RIR complex determined by NMR spectroscopy revealed a structural basis for the relatively high affinity of this interaction. The unexpected discovery of PolD3-RIR motif suggests a mechanism of "inserter" to "extender" DNA polymerase switch upon Rev1/Polζ-dependent TLS, in which the PolD3-RIR binding to the Rev1-CT (i) helps displace the "inserter" Polη, Polι, or Polκ from its complex with Rev1, and (ii) facilitates assembly of the four-subunit "extender" Polζ through simultaneous interaction of Rev1-CT with Rev7 and PolD3 subunits.
- Department of Molecular Biology and Biophysics, University of Connecticut Health Center , Farmington, Connecticut 06030, United States.
Organizational Affiliation: