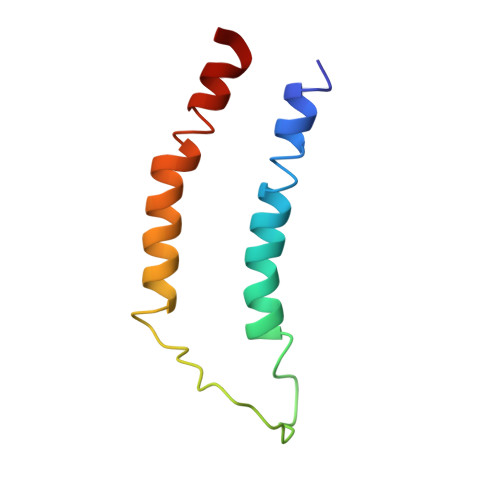
Structural Characterization of an LPA1 Second Extracellular Loop Mimetic with a Self-Assembling Coiled-Coil Folding Constraint.
Young, J.K., Clayton, B.T., Kikonyogo, A., Pham, T.C., Parrill, A.L.(2013) Int J Mol Sci 14: 2788-2807
- PubMed: 23434648
- DOI: https://doi.org/10.3390/ijms14022788
- Primary Citation of Related Structures:
2LQ4 - PubMed Abstract:
G protein-coupled receptor (GPCR) structures are of interest as a means to understand biological signal transduction and as tools for therapeutic discovery. The growing number of GPCR crystal structures demonstrates that the extracellular loops (EL) connecting the membrane-spanning helices show tremendous structural variability relative to the more structurally-conserved seven transmembrane α-helical domains. The EL of the LPA(1) receptor have not yet been conclusively resolved, and bear limited sequence identity to known structures. This study involved development of a peptide to characterize the intrinsic structure of the LPA(1) GPCR second EL. The loop was embedded between two helices that assemble into a coiled-coil, which served as a receptor-mimetic folding constraint (LPA(1)-CC-EL2 peptide). The ensemble of structures from multi-dimensional NMR experiments demonstrated that a robust coiled-coil formed without noticeable deformation due to the EL2 sequence. In contrast, the EL2 sequence showed well-defined structure only near its C-terminal residues. The NMR ensemble was combined with a computational model of the LPA(1) receptor that had previously been validated. The resulting hybrid models were evaluated using docking. Nine different hybrid models interacted with LPA 18:1 as expected, based on prior mutagenesis studies, and one was additionally consistent with antagonist affinity trends.
- Department of Chemistry and Computational Research on Materials Institute, The University of Memphis, Memphis, TN 38152-3550, USA. aparrill@memphis.edu.
Organizational Affiliation: