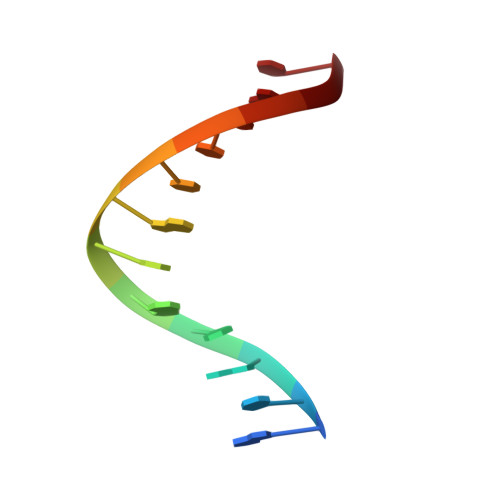
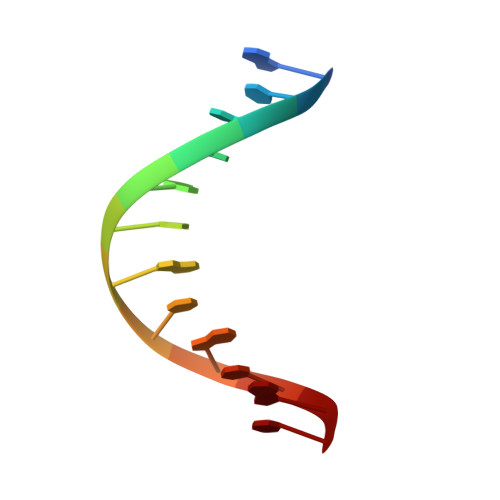
Structure of (5'S)-8,5'-Cyclo-2'-deoxyguanosine in DNA.
Huang, H., Das, R.S., Basu, A.K., Stone, M.P.(2011) J Am Chem Soc 133: 20357-20368
- PubMed: 22103478
- DOI: https://doi.org/10.1021/ja207407n
- Primary Citation of Related Structures:
2LFA, 2LG0 - PubMed Abstract:
Diastereomeric 8,5'-cyclopurine 2'-deoxynucleosides, containing a covalent bond between the deoxyribose and the purine base, represent an important class of DNA damage induced by ionizing radiation. The 8,5'-cyclo-2'-deoxyguanosine lesion (cdG) has been recently reported to be a strong block of replication and highly mutagenic in Escherichia coli. The 8,5'-cyclopurine-2'-deoxyriboses are suspected to play a role in the etiology of neurodegeneration in xeroderma pigmentosum patients. These lesions cannot be repaired by base excision repair, but they are substrates for nucleotide excision repair. The structure of an oligodeoxynucleotide duplex containing a site-specific S-cdG lesion placed opposite dC in the complementary strand was obtained by molecular dynamics calculations restrained by distance and dihedral angle restraints obtained from NMR spectroscopy. The S-cdG deoxyribose exhibited the O4'-exo (west) pseudorotation. Significant perturbations were observed for the β, γ, and χ torsion angles of the S-cdG nucleoside. Watson-Crick base pairing was conserved at the S-cdG·dC pair. However, the O4'-exo pseudorotation of the S-cdG deoxyribose perturbed the helical twist and base pair stacking at the lesion site and the 5'-neighbor dC·dG base pair. Thermodynamic destabilization of the duplex measured by UV melting experiments correlated with base stacking and structural perturbations involving the modified S-cdG·dC and 3'- neighbor dT·dA base pairs. These perturbations may be responsible for both the genotoxicity of this lesion and its ability to be recognized by nucleotide excision repair.
- Department of Chemistry, Center in Molecular Toxicology, Center for Structural Biology, and the Vanderbilt-Ingram Cancer Center, Vanderbilt University , Nashville, Tennessee 37235, USA.
Organizational Affiliation: