Atomic Structure of the kinetochore Spc24p/Spc25p globular domain reveals a novel fold
Wei, R.R., Harrison, S.C., Schnell, J.R., Chou, J.J.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Hypothetical 25.2 kDa protein in AFG3-SEB2 intergenic region | 90 | Saccharomyces cerevisiae | Mutation(s): 2 Gene Names: YER018C | 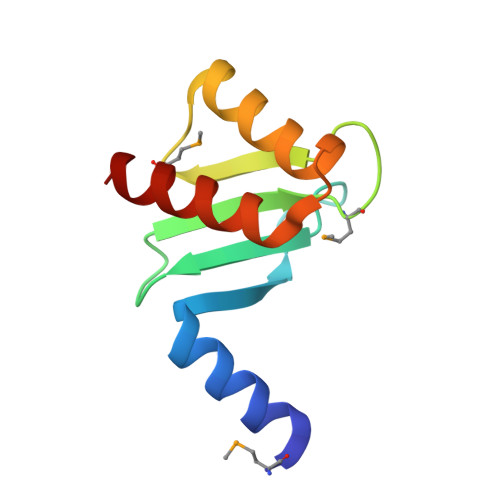 | |
UniProt | |||||
Find proteins for P40014 (Saccharomyces cerevisiae (strain ATCC 204508 / S288c)) Explore P40014 Go to UniProtKB: P40014 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P40014 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Hypothetical 24.6 kDa protein in ILV2-ADE17 intergenic region | 64 | Saccharomyces cerevisiae | Mutation(s): 0 Gene Names: YMR117C, YM9718.16C | 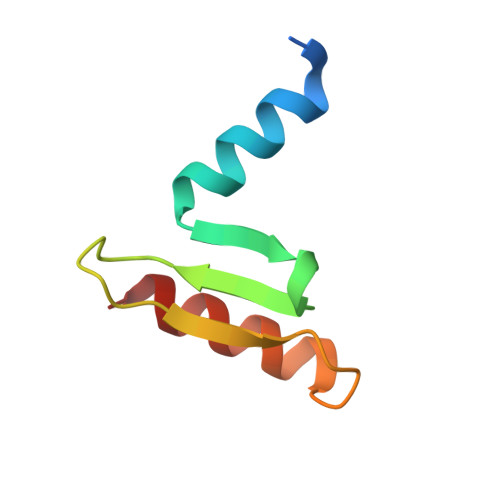 | |
UniProt | |||||
Find proteins for Q04477 (Saccharomyces cerevisiae (strain ATCC 204508 / S288c)) Explore Q04477 Go to UniProtKB: Q04477 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q04477 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| PO4 Query on PO4 | D [auth A] | PHOSPHATE ION O4 P NBIIXXVUZAFLBC-UHFFFAOYSA-K |  | ||
| NA Query on NA | C [auth A] | SODIUM ION Na FKNQFGJONOIPTF-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| MSE Query on MSE | A | L-PEPTIDE LINKING | C5 H11 N O2 Se |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 84.907 | α = 90 |
| b = 84.907 | β = 90 |
| c = 93.195 | γ = 90 |
| Software Name | Purpose |
|---|---|
| HKL-2000 | data collection |
| SCALEPACK | data scaling |
| SOLVE | phasing |
| REFMAC | refinement |
| HKL-2000 | data reduction |