Crystal Structure of Phosphoribosyl-ATP Pyrophosphatase from Chromobacterium violaceum. NESG Target CVR7.
Benach, J., Forouhar, F., Kuzin, A.P., Abashidze, M., Vorobiev, S.M., Rong, X., Acton, T.B., Montelione, G.T., Hunt, J.F.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Phosphoribosyl-ATP pyrophosphatase | 116 | Chromobacterium violaceum | Mutation(s): 4 Gene Names: hisE EC: 3.6.1.31 | 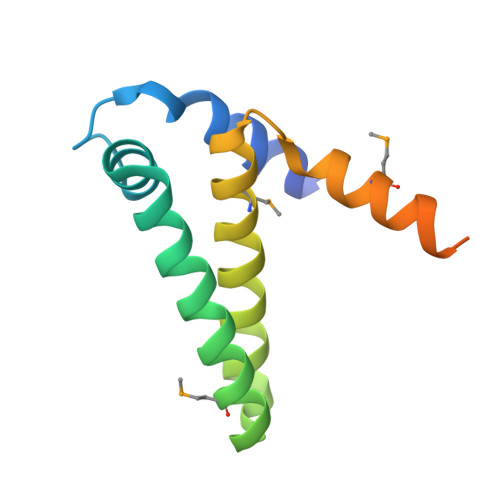 | |
UniProt | |||||
Find proteins for Q7P0E6 (Chromobacterium violaceum (strain ATCC 12472 / DSM 30191 / JCM 1249 / CCUG 213 / NBRC 12614 / NCIMB 9131 / NCTC 9757 / MK)) Explore Q7P0E6 Go to UniProtKB: Q7P0E6 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q7P0E6 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| MSE Query on MSE | A, B, C, D, E A, B, C, D, E, F, G, H, I, J, K, L | L-PEPTIDE LINKING | C5 H11 N O2 Se |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 97.938 | α = 90 |
| b = 67.544 | β = 92.45 |
| c = 113.512 | γ = 90 |
| Software Name | Purpose |
|---|---|
| HKL-2000 | data collection |
| SCALEPACK | data scaling |
| SOLVE | phasing |
| CNS | refinement |
| HKL-2000 | data reduction |