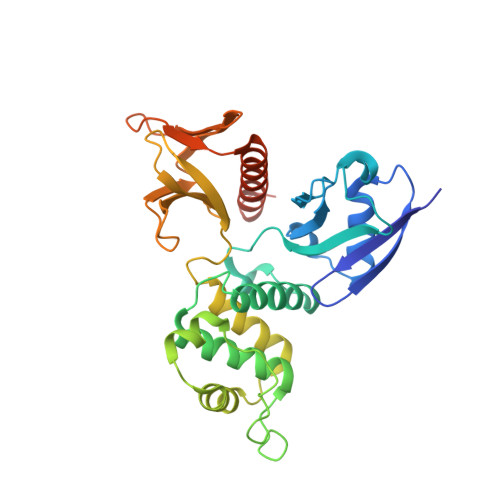
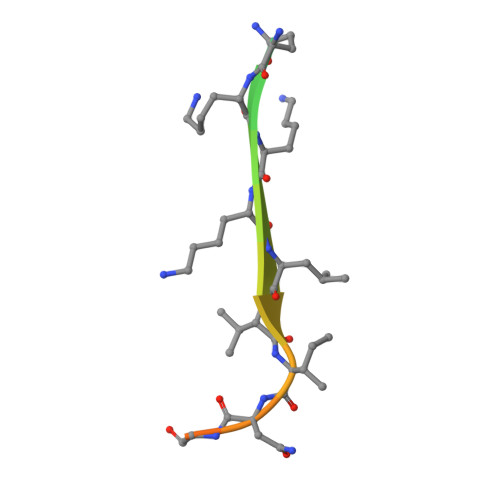
Structural basis for CD44 recognition by ERM proteins
Mori, T., Kitano, K., Terawaki, S., Maesaki, R., Fukami, Y., Hakoshima, T.(2008) J Biological Chem 283: 29602-29612
- PubMed: 18753140
- DOI: https://doi.org/10.1074/jbc.M803606200
- Primary Citation of Related Structures:
2ZPY - PubMed Abstract:
CD44 is an important adhesion molecule that functions as the major hyaluronan receptor which mediates cell adhesion and migration in a variety of physiological and pathological processes. Although full activity of CD44 requires binding to ERM (ezrin/radixin/moesin) proteins, the CD44 cytoplasmic region, consisting of 72 amino acid residues, lacks the Motif-1 consensus sequence for ERM binding found in intercellular adhesion molecule (ICAM)-2 and other adhesion molecules of the immunoglobulin superfamily. Ultracentrifugation sedimentation studies and circular dichroism measurements revealed an extended monomeric form of the cytoplasmic peptide in solution. The crystal structure of the radixin FERM domain complexed with a CD44 cytoplasmic peptide reveals that the KKKLVIN sequence of the peptide forms a beta strand followed by a short loop structure that binds subdomain C of the FERM domain. Like Motif-1 binding, the CD44 beta strand binds the shallow groove between strand beta5C and helix alpha1C and augments the beta sheet beta5C-beta7C from subdomain C. Two hydrophobic CD44 residues, Leu and Ile, are docked into a hydrophobic pocket with the formation of hydrogen bonds between Asn of the CD44 short loop and loop beta4C-beta5C from subdomain C. This binding mode resembles that of NEP (neutral endopeptidase 24.11) rather than ICAM-2. Our results reveal a characteristic versatility of peptide recognition by the FERM domains from ERM proteins, suggest a possible mechanism by which the CD44 tail is released from the cytoskeleton for nuclear translocation by regulated intramembrane proteolysis, and provide a structural basis for Smad1 interactions with activated CD44 bound to ERM protein.
- Structural Biology Laboratory, Nara Institute of Science and Technology, 8916-5 Takayama, Ikoma, Nara 630-0192, Japan.
Organizational Affiliation: