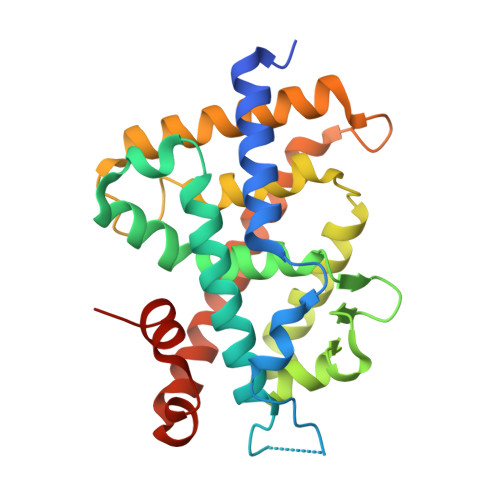
Structure of the ligand-binding domain of rat VDR in complex with the nonsecosteroidal vitamin D3 analogue YR301
Kakuda, S., Okada, K., Eguchi, H., Takenouchi, K., Hakamata, W., Kurihara, M., Takimoto-Kamimura, M.(2008) Acta Crystallogr Sect F Struct Biol Cryst Commun 64: 970-973
- PubMed: 18997319
- DOI: https://doi.org/10.1107/S1744309108026754
- Primary Citation of Related Structures:
2ZFX - PubMed Abstract:
Vitamin D receptor (VDR) is a ligand-inducible hormone receptor that mediates 1alpha,25(OH)(2)D(3) action, regulating calcium and phosphate metabolism, induces potent cell differentiation activity and has immunosuppressive effects. Analogues of 1alpha,25(OH)(2)D(3) have been used clinically for some years. However, the risk of potential side effects limits the use of these substances. LG190178 is a novel nonsecosteroidal ligand for VDR. (2S)-3-[4-(3-{4-[(2R)-2-hydroxy-3,3-dimethylbutoxy]-3-methylphenyl}pentan-3-yl)-2-methylphenoxy] propane-1,2-diol (YR301) is the only one of the four evaluated stereoisomers of LG190178 to have strong activity. To understand the strong activity of YR301, the crystal structure of YR301 complexed with the rat VDR ligand-binding domain (VDR LBD) was solved at 2.0 A resolution and compared with the structure of the VDR LBD-1alpha,25(OH)(2)D(3) complex. YR301 and 1alpha,25(OH)(2)D(3) share the same position and the diethylmethyl group occupies a similar space to the C and D rings of 1alpha,25(OH)(2)D(3). YR301 has two characteristic hydroxyl groups which contribute to its potent activity. The first is 2'-OH, which forms hydrogen bonds to the NE2 atoms of both His301 and His393. The other is 2-OH, which interacts with Ser233 OG and Arg270 NH1. These two hydroxyl groups of YR301 correspond exactly to 25-OH and 1-OH, respectively, of 1alpha,25(OH)(2)D(3). The terminal hydroxyl group (3-OH) of YR301 is directly hydrogen bonded to Arg270 and also interacts indirectly with Tyr232 OH and the backbone NH of Asp144 via water molecules. Additional derivatization of the terminal hydroxyl group using the positions of the water molecules might be useful for the design of more potent compounds.
- Teijin Institute for Biomedical Research, Japan. s.kakuda@teijin.co.jp
Organizational Affiliation: