Structural Insight Into the Giant Ca(2+)-Binding Adhesin Siie: Implications for the Adhesion of Salmonella Enterica to Polarized Epithelial Cells.
Griessl, M.H., Schmid, B., Kassler, K., Braunsmann, C., Ritter, R., Barlag, B., Stierhof, Y., Sturm, K.U., Danzer, C., Wagner, C., Schaffer, T.E., Sticht, H., Hensel, M., Muller, Y.A.(2013) Structure 21: 741
- PubMed: 23562396
- DOI: https://doi.org/10.1016/j.str.2013.02.020
- Primary Citation of Related Structures:
2YN3, 2YN5 - PubMed Abstract:
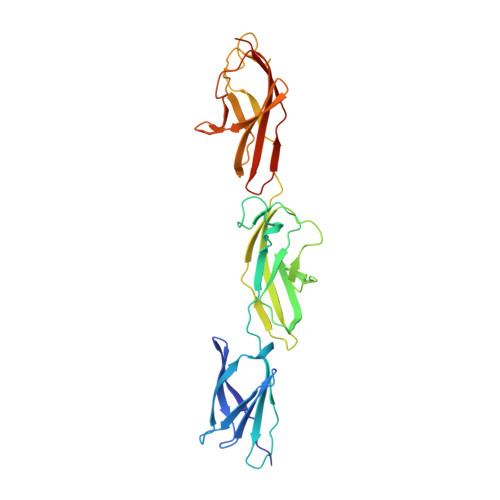
SiiE from Salmonella enterica is a giant 5,559-residue-long nonfimbrial adhesin that is secreted by a type 1 secretion system (T1SS) and initiates bacterial adhesion to polarized host cells. Structural insight has been gained into the 53 bacterial Ig-like (BIg) domains of SiiE, which account for 94% of the entire SiiE sequence. The crystal structure of a fragment comprising BIg domains 50 to 52 of SiiE reveals the BIg domain architecture and highlights two types of SiiE-specific Ca²⁺-binding sites. Sequence homology considerations suggest that full-length SiiE interacts with more than 100 Ca²⁺ ions. Molecular dynamics simulations and single-molecule imaging indicate that Ca²⁺ binding confers SiiE with a rigid 200 nm rod-like habitus that is required to reach out beyond the Salmonella lipopolysaccharide layer and to promote adhesion to host cells. The crystal structure suggests plausible routes for the establishment of the initial contact between Salmonella and host cells.
- Lehrstuhl für Biotechnik, Department of Biology, Friedrich-Alexander-University of Erlangen-Nuremberg, 91054 Erlangen, Germany.
Organizational Affiliation: