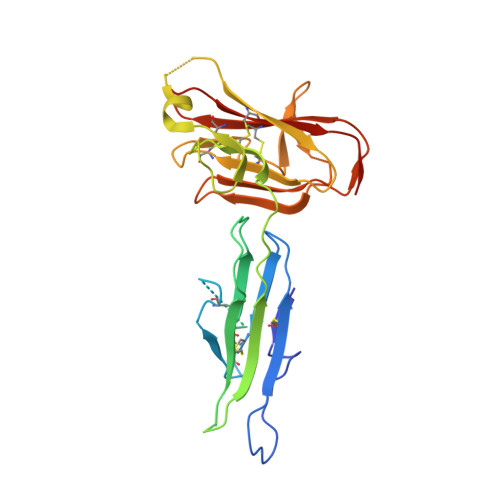
Structural and Biochemical Characterization of Plasmodium Falciparum 12 (Pf12) Reveals a Unique Inter-Domain Organization and the Potential for an Antiparallel Arrangement with Pf41
Tonkin, M.L., Arredondo, S.A., Loveless, B.C., Serpa, J.J., Makepeace, K.A.T., Sundar, N., Petrotchenko, E.V., Miller, L.H., Grigg, M.E., Boulanger, M.J.(2013) J Biological Chem 288: 12805
- PubMed: 23511632
- DOI: https://doi.org/10.1074/jbc.M113.455667
- Primary Citation of Related Structures:
2YMO - PubMed Abstract:
Plasmodium falciparum is the most devastating agent of human malaria. A major contributor to its virulence is a complex lifecycle with multiple parasite forms, each presenting a different repertoire of surface antigens. Importantly, members of the 6-Cys s48/45 family of proteins are found on the surface of P. falciparum in every stage, and several of these antigens have been investigated as vaccine targets. Pf12 is the archetypal member of the 6-Cys protein family, containing just two s48/45 domains, whereas other members have up to 14 of these domains. Pf12 is strongly recognized by immune sera from naturally infected patients. Here we show that Pf12 is highly conserved and under purifying selection. Immunofluorescence data reveals a punctate staining pattern with an apical organization in late schizonts. Together, these data are consistent with an important functional role for Pf12 in parasite-host cell attachment or invasion. To infer the structural and functional diversity between Pf12 and the other 11 6-Cys domain proteins, we solved the 1.90 Å resolution crystal structure of the Pf12 ectodomain. Structural analysis reveals a unique organization between the membrane proximal and membrane distal domains and clear homology with the SRS-domain containing proteins of Toxoplasma gondii. Cross-linking and mass spectrometry confirm the previously identified Pf12-Pf41 heterodimeric complex, and analysis of individual cross-links supports an unexpected antiparallel organization. Collectively, the localization and structure of Pf12 and details of its interaction with Pf41 reveal important insight into the structural and functional properties of this archetypal member of the 6-Cys protein family.
- Biochemistry and Microbiology, University of Victoria, Victoria, British Columbia, Canada V8W 3P6.
Organizational Affiliation: