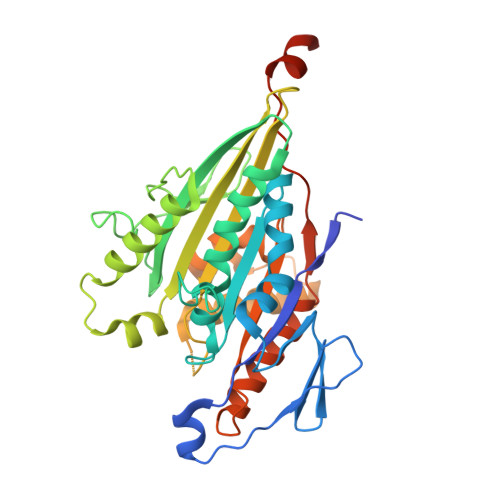
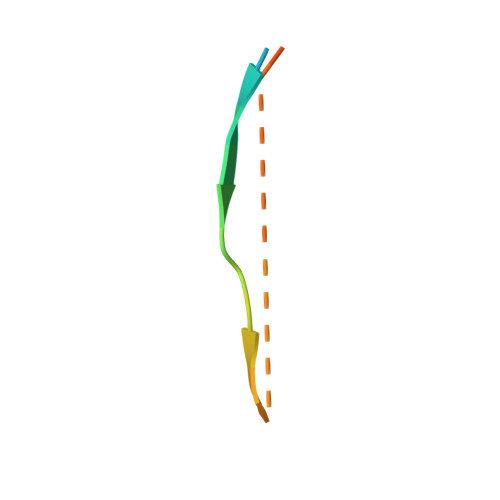
The Structure of the Kinesin-1 Motor-Tail Complex Reveals the Mechanism of Autoinhibition.
Kaan, H.Y.K., Hackney, D.D., Kozielski, F.(2011) Science 333: 883
- PubMed: 21836017
- DOI: https://doi.org/10.1126/science.1204824
- Primary Citation of Related Structures:
2Y5W, 2Y65 - PubMed Abstract:
When not transporting cargo, kinesin-1 is autoinhibited by binding of a tail region to the motor domains, but the mechanism of inhibition is unclear. We report the crystal structure of a motor domain dimer in complex with its tail domain at 2.2 angstroms and compare it with a structure of the motor domain alone at 2.7 angstroms. These structures indicate that neither an induced conformational change nor steric blocking is the cause of inhibition. Instead, the tail cross-links the motor domains at a second position, in addition to the coiled coil. This "double lockdown," by cross-linking at two positions, prevents the movement of the motor domains that is needed to undock the neck linker and release adenosine diphosphate. This autoinhibition mechanism could extend to some other kinesins.
- The Beatson Institute for Cancer Research, Switchback Road, Bearsden, Glasgow G61 1BD, Scotland, UK.
Organizational Affiliation: