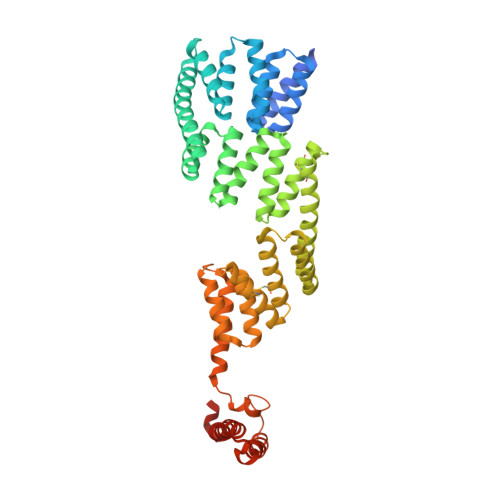
The Crystal Structure of the Human Co-Chaperone P58Ipk
Svard, M., Biterova, E.I., Bourhis, J.-M., Guy, J.E.(2011) PLoS One 6: 22337
- PubMed: 21799829
- DOI: https://doi.org/10.1371/journal.pone.0022337
- Primary Citation of Related Structures:
2Y4T, 2Y4U - PubMed Abstract:
P58(IPK) is one of the endoplasmic reticulum- (ER-) localised DnaJ (ERdj) proteins which interact with the chaperone BiP, the mammalian ER ortholog of Hsp70, and are thought to contribute to the specificity and regulation of its diverse functions. P58(IPK), expression of which is upregulated in response to ER stress, has been suggested to act as a co-chaperone, binding un- or misfolded proteins and delivering them to BiP. In order to give further insights into the functions of P58(IPK), and the regulation of BiP by ERdj proteins, we have determined the crystal structure of human P58(IPK) to 3.0 Å resolution using a combination of molecular replacement and single wavelength anomalous diffraction. The structure shows the human P58(IPK) monomer to have a very elongated overall shape. In addition to the conserved J domain, P58(IPK) contains nine N-terminal tetratricopeptide repeat motifs, divided into three subdomains of three motifs each. The J domain is attached to the C-terminal end via a flexible linker, and the structure shows the conserved Hsp70-binding histidine-proline-aspartate (HPD) motif to be situated on the very edge of the elongated protein, 100 Å from the putative binding site for unfolded protein substrates. The residues that comprise the surface surrounding the HPD motif are highly conserved in P58(IPK) from other organisms but more varied between the human ERdj proteins, supporting the view that their regulation of different BiP functions is facilitated by differences in BiP-binding.
- Department of Medical Biochemistry and Biophysics, Karolinska Institutet, Stockholm, Sweden.
Organizational Affiliation: