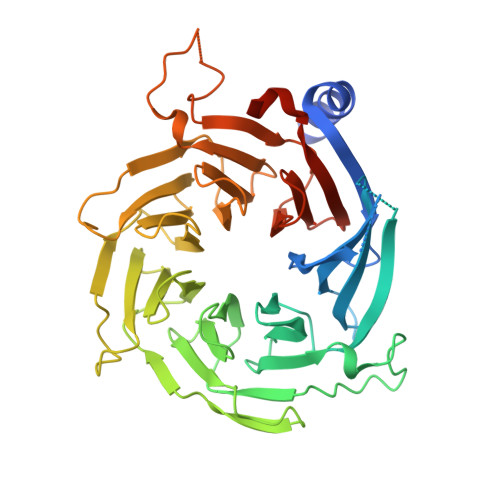
Insights Into Association of the Nurd Complex with Fog-1 from the Crystal Structure of an Rbap48-Fog- 1 Complex.
Lejon, S., Thong, S.Y., Murthy, A., Alqarni, S., Murzina, N.V., Blobel, G.A., Laue, E.D., Mackay, J.P.(2011) J Biological Chem 286: 1196
- PubMed: 21047798
- DOI: https://doi.org/10.1074/jbc.M110.195842
- Primary Citation of Related Structures:
2XU7 - PubMed Abstract:
Chromatin-modifying complexes such as the NuRD complex are recruited to particular genomic sites by gene-specific nuclear factors. Overall, however, little is known about the molecular basis for these interactions. Here, we present the 1.9 Å resolution crystal structure of the NuRD subunit RbAp48 bound to the 15 N-terminal amino acids of the GATA-1 cofactor FOG-1. The FOG-1 peptide contacts a negatively charged binding pocket on top of the RbAp48 β-propeller that is distinct from the binding surface used by RpAp48 to contact histone H4. We further show that RbAp48 interacts with the NuRD subunit MTA-1 via a surface that is distinct from its FOG-binding pocket, providing a first glimpse into the way in which NuRD assembly facilitates interactions with cofactors. Our RbAp48·FOG-1 structure provides insight into the molecular determinants of FOG-1-dependent association with the NuRD complex and into the links between transcription regulation and nucleosome remodeling.
- Department of Biochemistry, University of Cambridge, Cambridge CB2 1GA, United Kingdom.
Organizational Affiliation: