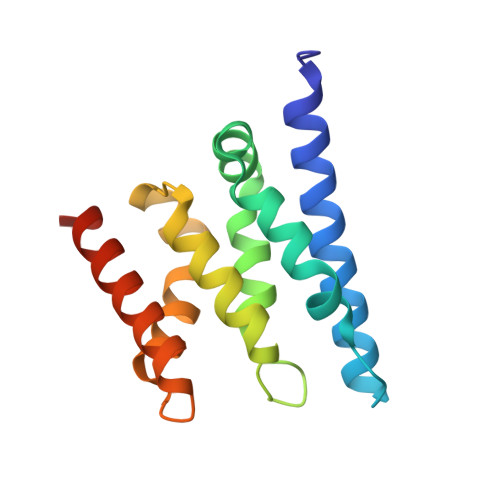
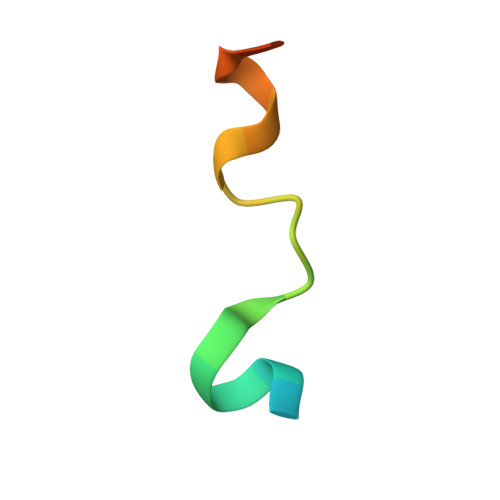
The Structure of an Iws1/Spt6 Complex Reveals an Interaction Domain Conserved in Tfiis, Elongin a and Med26
Diebold, M.-L., Koch, M., Loeliger, E., Cura, V., Winston, F., Cavarelli, J., Romier, C.(2010) EMBO J 29: 3979
- PubMed: 21057455
- DOI: https://doi.org/10.1038/emboj.2010.272
- Primary Citation of Related Structures:
2XPL, 2XPN, 2XPO, 2XPP - PubMed Abstract:
Binding of elongation factor Spt6 to Iws1 provides an effective means for coupling eukaryotic mRNA synthesis, chromatin remodelling and mRNA export. We show that an N-terminal region of Spt6 (Spt6N) is responsible for interaction with Iws1. The crystallographic structures of Encephalitozoon cuniculi Iws1 and the Iws1/Spt6N complex reveal two conserved binding subdomains in Iws1. The first subdomain (one HEAT repeat; HEAT subdomain) is a putative phosphoprotein-binding site most likely involved in an Spt6-independent function of Iws1. The second subdomain (two ARM repeats; ARM subdomain) specifically recognizes a bipartite N-terminal region of Spt6. Mutations that alter this region of Spt6 cause severe phenotypes in vivo. Importantly, the ARM subdomain of Iws1 is conserved in several transcription factors, including TFIIS, Elongin A and Med26. We show that the homologous region in yeast TFIIS enables this factor to interact with SAGA and the Mediator subunits Spt8 and Med13, suggesting the molecular basis for TFIIS recruitment at promoters. Taken together, our results provide new structural information about the Iws1/Spt6 complex and reveal a novel interaction domain used for the formation of transcription networks.
- Département de Biologie et Génomique Structurales, IGBMC (Institut de Génétique et Biologie Moléculaire et Cellulaire), UDS, CNRS, INSERM, Illkirch Cedex, France.
Organizational Affiliation: