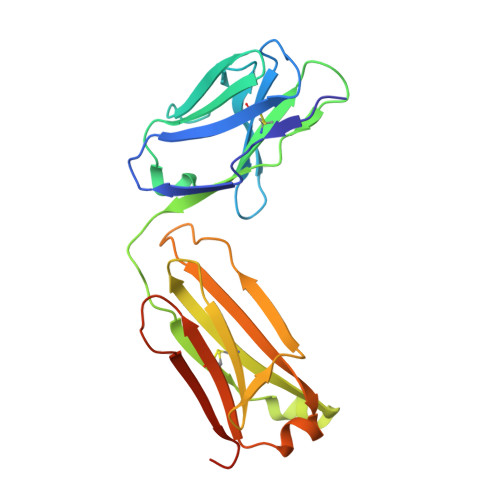
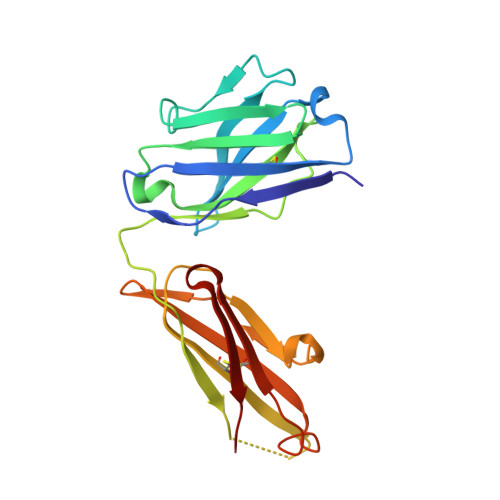
Structure of the Fab Fragment of the Anti-Murine Egfr Antibody 7A7 and Exploration of its Receptor Binding Site.
Talavera, A., Mackenzie, J., Garrido, G., Friemann, R., Lopez-Requena, A., Moreno, E., Krengel, U.(2011) Mol Immunol 48: 1578
- PubMed: 21592580
- DOI: https://doi.org/10.1016/j.molimm.2011.03.016
- Primary Citation of Related Structures:
2XKN - PubMed Abstract:
The EGF receptor is an important target of cancer immunotherapies. The 7A7 monoclonal antibody has been raised against the murine EGFR, but it cross-reacts with the human receptor. The results from experiments using immune-competent mice can therefore, in principle, be extrapolated to the corresponding scenario in humans. In this work we report the crystal structure of the 7A7 Fab at an effective resolution of 1.4Å. The antibody binding site comprises a deep pocket, located at the interface between the light and heavy chains, with major contributions from CDR loops H1, H2, H3 and L1. Binding experiments show that 7A7 recognizes a site on the EGFR extracellular domain that is not accessible in its most stable conformations, but that becomes exposed upon treatment with a tyrosine kinase inhibitor. This suggests a recognition mechanism similar to that proposed for mAb 806.
- Department of Chemistry, University of Oslo, PO Box 1033 Blindern, NO-0315 Oslo, Norway. talavera@cim.sld.cu
Organizational Affiliation: