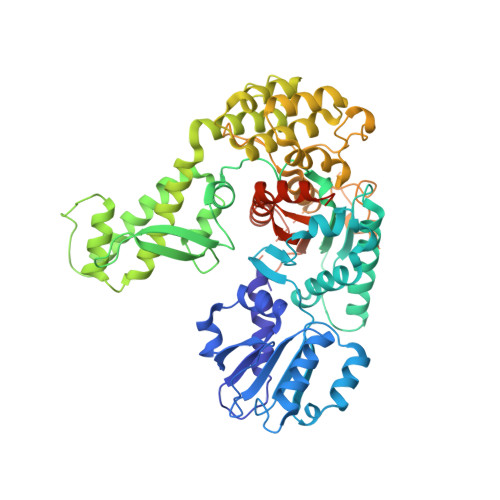
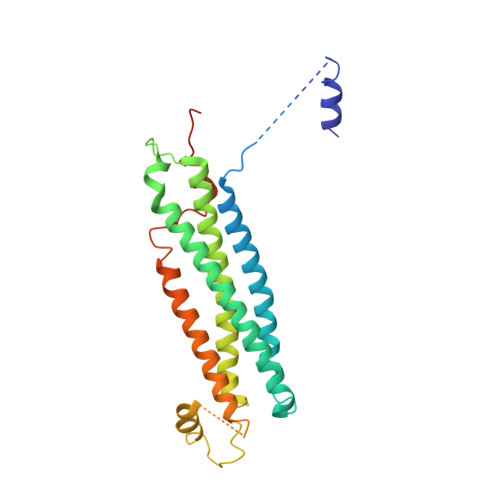
Primordial Neurosecretory Apparatus Identified in the Choanoflagellate Monosiga Brevicollis.
Burkhardt, P., Stegmann, C.M., Cooper, B., Kloepper, T.H., Imig, C., Varoqueaux, F., Wahl, M.C., Fasshauer, D.(2011) Proc Natl Acad Sci U S A 108: 15264
- PubMed: 21876177
- DOI: https://doi.org/10.1073/pnas.1106189108
- Primary Citation of Related Structures:
2XHE - PubMed Abstract:
SNARE protein-driven secretion of neurotransmitters from synaptic vesicles is at the center of neuronal communication. In the absence of the cytosolic protein Munc18-1, synaptic secretion comes to a halt. Although it is believed that Munc18-1 orchestrates SNARE complexes, its mode of action is still a matter of debate. In particular, it has been challenging to clarify the role of a tight Munc18/syntaxin 1 complex, because this interaction interferes strongly with syntaxin's ability to form a SNARE complex. In this complex, two regions of syntaxin, the N-peptide and the remainder in closed conformation, bind to Munc18 simultaneously. Until now, this binary complex has been reported for neuronal tissues only, leading to the hypothesis that it might be a specialization of the neuronal secretion apparatus. Here we aimed, by comparing the core secretion machinery of the unicellular choanoflagellate Monosiga brevicollis with that of animals, to reconstruct the ancestral function of the Munc18/syntaxin1 complex. We found that the Munc18/syntaxin 1 complex from M. brevicollis is structurally and functionally highly similar to the vertebrate complex, suggesting that it constitutes a fundamental step in the reaction pathway toward SNARE assembly. We thus propose that the primordial secretion machinery of the common ancestor of choanoflagellates and animals has been co-opted for synaptic roles during the rise of animals.
- Research Group Structural Biochemistry, Department of Neurobiology, and Research Group X-Ray Crystallography, Max Planck Institute for Biophysical Chemistry, 37077 Göttingen, Germany.
Organizational Affiliation: