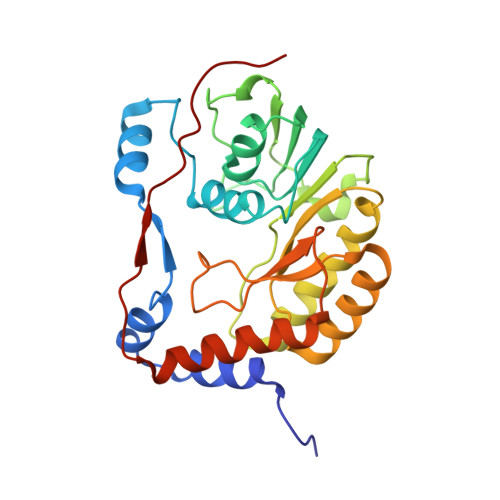
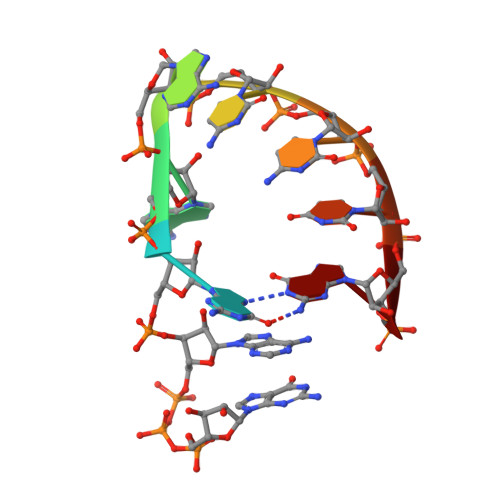
Crystal Structure of the Dengue Virus Methyltransferase Bound to a 5'-Capped Octameric RNA
Yap, L.J., Luo, D.H., Chung, K.Y., Lim, S.P., Bodenreider, C., Noble, C., Shi, P.Y., Lescar, J.(2010) PLoS One 5: 12836
- PubMed: 20862256
- DOI: https://doi.org/10.1371/journal.pone.0012836
- Primary Citation of Related Structures:
2XBM - PubMed Abstract:
The N-terminal domain of the flavivirus NS5 protein functions as a methyltransferase (MTase). It sequentially methylates the N7 and 2'-O positions of the viral RNA cap structure (GpppA→(7me)GpppA→(7me)GpppA(2'-O-me)). The same NS5 domain could also have a guanylyltransferase activity (GTP+ppA-RNA→GpppA). The mechanism by which this protein domain catalyzes these three distinct functions is currently unknown. Here we report the crystallographic structure of DENV-3 MTase in complex with a 5'-capped RNA octamer (G(ppp)AGAACCUG) at a resolution of 2.9 A. Two RNA octamers arranged as kissing loops are encircled by four MTase monomers around a 2-fold non-crystallography symmetry axis. Only two of the four monomers make direct contact with the 5' end of RNA. The RNA structure is stabilised by the formation of several intra and intermolecular base stacking and non-canonical base pairs. The structure may represent the product of guanylylation of the viral genome prior to the subsequent methylation events that require repositioning of the RNA substrate to reach to the methyl-donor sites. The crystal structure provides a structural explanation for the observed trans-complementation of MTases with different methylation defects.
- School of Biological Sciences, Nanyang Technological University, Singapore, Singapore.
Organizational Affiliation: