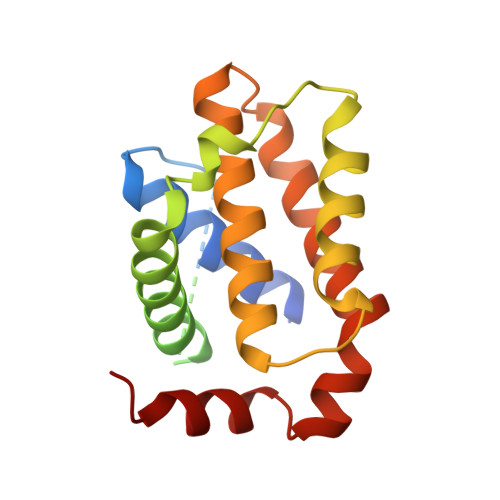
Evidence that Inhibition of Bax Activation by Bcl- 2 Involves its Tight and Preferential Interaction with the Bh3 Domain of Bax.
Ku, B., Liang, C., Jung, J.U., Oh, B.H.(2011) Cell Res 21: 627
- PubMed: 21060336
- DOI: https://doi.org/10.1038/cr.2010.149
- Primary Citation of Related Structures:
2XA0 - PubMed Abstract:
Interactions between the BCL-2 family proteins determine the cell's fate to live or die. How they interact with each other to regulate apoptosis remains as an unsettled central issue. So far, the antiapoptotic BCL-2 proteins are thought to interact with BAX weakly, but the physiological significance of this interaction has been vague. Herein, we show that recombinant BCL-2 and BCL-w interact potently with a BCL-2 homology (BH) 3 domain-containing peptide derived from BAX, exhibiting the dissociation constants of 15 and 23 nM, respectively. To clarify the basis for this strong interaction, we determined the three-dimensional structure of a complex of BCL-2 with a BAX peptide spanning its BH3 domain. It revealed that their interactions extended beyond the canonical BH3 domain and involved three nonconserved charged residues of BAX. A novel BAX variant, containing the alanine substitution of these three residues, had greatly impaired affinity for BCL-2 and BCL-w, but was otherwise indistinguishable from wild-type BAX. Critically, the apoptotic activity of the BAX variant could not be restrained by BCL-2 and BCL-w, pointing that the observed tight interactions are critical for regulating BAX activation. We also comprehensively quantified the binding affinities between the three BCL-2 subfamily proteins. Collectively, the data show that due to the high affinity of BAX for BCL-2, BCL-w and A1, and of BAK for BCL-X(L), MCL-1 and A1, only a subset of BH3-only proteins, commonly including BIM, BID and PUMA, could be expected to free BAX or BAK from the antiapoptotic BCL-2 proteins to elicit apoptosis.
- Department of Biological Sciences, KAIST Institute for the Biocentury, Daejeon, Korea.
Organizational Affiliation: