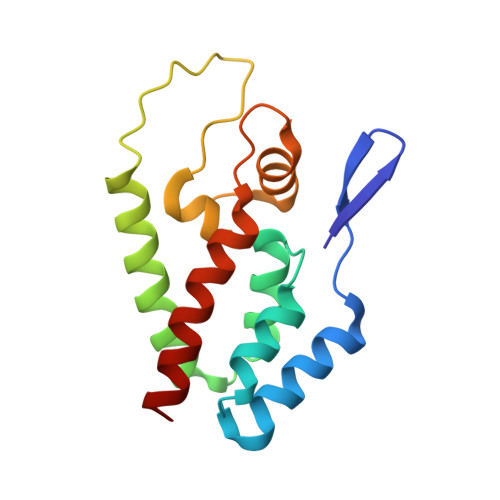
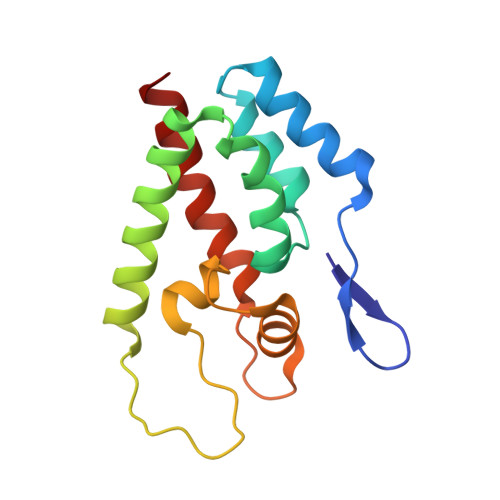
Active Site Remodelling Switches HIV Specificity of Antiretroviral Trimcyp
Price, A.J., Marzetta, F., Lammers, M., Ylinen, L.M.J., Schaller, T., Wilson, S.J., Towers, G.J., James, L.C.(2009) Nat Struct Mol Biol 16: 1036
- PubMed: 19767750
- DOI: https://doi.org/10.1038/nsmb.1667
- Primary Citation of Related Structures:
2WLV, 2WLW - PubMed Abstract:
TRIMCyps are primate antiretroviral proteins that potently inhibit HIV replication. Here we describe how rhesus macaque TRIMCyp (RhTC) has evolved to target and restrict HIV-2. We show that the ancestral cyclophilin A (CypA) domain of RhTC targets HIV-2 capsid with weak affinity, which is strongly increased in RhTC by two mutations (D66N and R69H) at the expense of HIV-1 binding. These mutations disrupt a constraining intramolecular interaction in CypA, triggering the complete restructuring (>16 A) of an active site loop. This new configuration discriminates between divergent HIV-1 and HIV-2 loop conformations mediated by capsid residue 88. Viral sensitivity to RhTC restriction can be conferred or abolished by mutating position 88. Furthermore, position 88 determines the susceptibility of naturally occurring HIV-1 sequences to restriction. Our results reveal the complex molecular, structural and thermodynamic changes that underlie the ongoing evolutionary race between virus and host.
- Protein and Nucleic Acid Chemistry Division, Medical Research Council Laboratory of Molecular Biology, Cambridge, UK.
Organizational Affiliation: