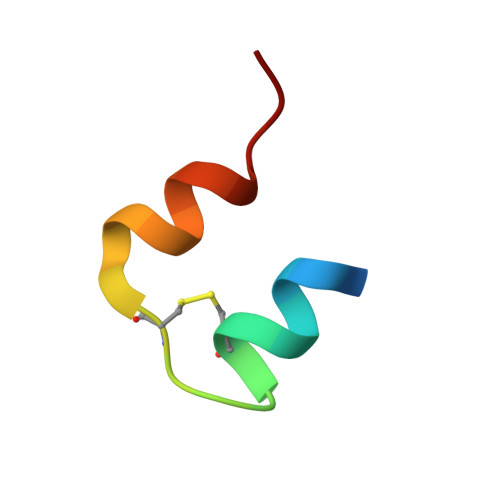
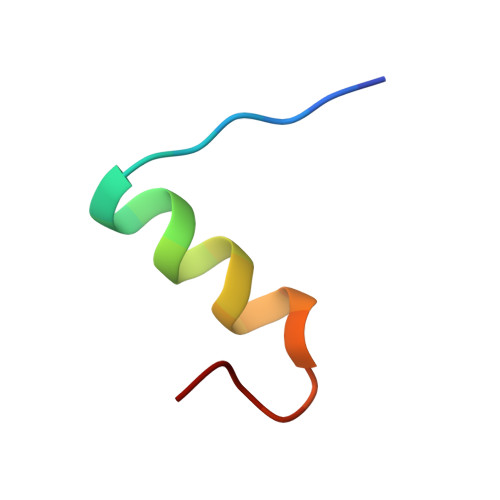
Structural and Biological Properties of the Drosophila Insulin-Like Peptide 5 Show Evolutionary Conservation.
Sajid, W., Kulahin, N., Schluckebier, G., Ribel, U., Henderson, H.R., Tatar, M., Hansen, B.F., Svendsen, A.M., Kiselyov, V.V., Norgaard, P., Wahlund, P., Brandt, J., Kohanski, R.A., Andersen, A.S., De Meyts, P.(2011) J Biological Chem 286: 661
- PubMed: 20974844
- DOI: https://doi.org/10.1074/jbc.M110.156018
- Primary Citation of Related Structures:
2WFU, 2WFV - PubMed Abstract:
We report the crystal structure of two variants of Drosophila melanogaster insulin-like peptide 5 (DILP5) at a resolution of 1.85 Å. DILP5 shares the basic fold of the insulin peptide family (T conformation) but with a disordered B-chain C terminus. DILP5 dimerizes in the crystal and in solution. The dimer interface is not similar to that observed in vertebrates, i.e. through an anti-parallel β-sheet involving the B-chain C termini but, in contrast, is formed through an anti-parallel β-sheet involving the B-chain N termini. DILP5 binds to and activates the human insulin receptor and lowers blood glucose in rats. It also lowers trehalose levels in Drosophila. Reciprocally, human insulin binds to the Drosophila insulin receptor and induces negative cooperativity as in the human receptor. DILP5 also binds to insect insulin-binding proteins. These results show high evolutionary conservation of the insulin receptor binding properties despite divergent insulin dimerization mechanisms.
- Receptor Systems Biology Laboratory, Insulin and Incretin Biology, Hagedorn Research Institute, 2820 Gentofte, Denmark.
Organizational Affiliation: