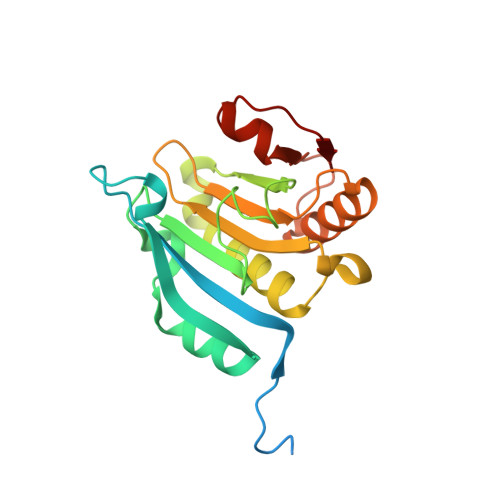
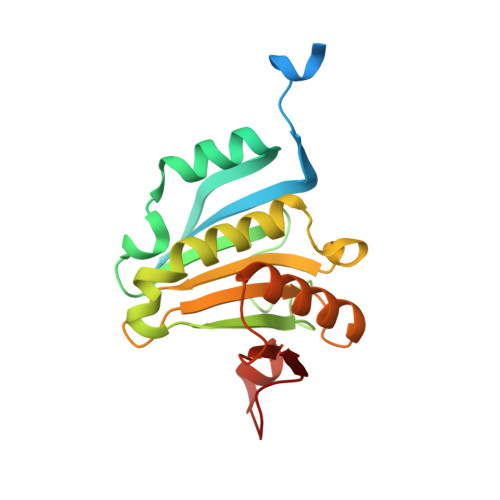
Crystallization of eIF4E complexed with eIF4GI peptide and glycerol reveals distinct structural differences around the cap-binding site.
Brown, C.J., Verma, C.S., Walkinshaw, M.D., Lane, D.P.(2009) Cell Cycle 8: 1905-1911
- PubMed: 19440045
- DOI: https://doi.org/10.4161/cc.8.12.8742
- Primary Citation of Related Structures:
2W97 - PubMed Abstract:
An X-ray crystal structure of the eIF4E peptide complex is described in which two such complexes are located in the asymmetric unit. One of these complexes has m(7)GTP bound in a conformation which has been observed in several eIF4E crystal structures, whilst the other complex is free of m(7)GTP and contains a unique glycerol. The two complexes show significant structural differences between each other in the cap-binding site. The glycerol bound structure shows a reorientation of the W102 side chain out of the cap-binding site, disordering of the W56 containing loop and rotation of the carboxyl side-chain of E103. This is accompanied by movement of the M101 side chain into a position where W56 in the m(7)GTP bound complex would otherwise occupy. Rotation of the W102 sidechain also displaces a structured water molecule to a new site. This novel conformation of eIF4E with glycerol bound is hypothesized to be an intermediate state between the apo and m(7)GTP bound forms of eIF4E. These insights should prove useful in the design of inhibitors of eIF4E for cancer therapy.
- Institute of Molecular and Cell Biology (A-STAR), Proteos, Singapore.
Organizational Affiliation: