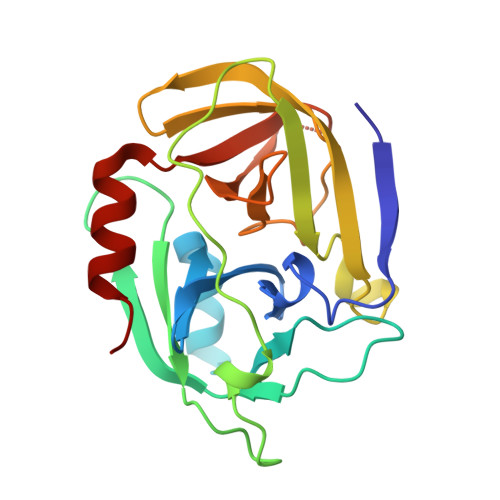
Structural and Functional Characterization of Spla, an Exclusively Specific Protease of Staphylococcus Aureus
Stec-Niemczyka, J., Pustelny, K., Kisielewska, M., Bista, M., Boulware, K.T., Stennicke, H.R., Thogersen, I.B., Daugherty, P.S., Enghild, J.J., Popowicz, G.M., Dubin, A., Potempa, J., Dubin, G.(2009) Biochem J 419: 555
- PubMed: 19175361
- DOI: https://doi.org/10.1042/BJ20081351
- Primary Citation of Related Structures:
2W7S, 2W7U - PubMed Abstract:
Staphylococcus aureus is a dangerous human pathogen whose antibiotic resistance is steadily increasing and no efficient vaccine is as yet available. This serious threat drives extensive studies on staphylococcal physiology and pathogenicity pathways, especially virulence factors. Spl (serine protease-like) proteins encoded by an operon containing up to six genes are a good example of poorly characterized secreted proteins probably involved in virulence. In the present study, we describe an efficient heterologous expression system for SplA and detailed biochemical and structural characterization of the recombinant SplA protease. The enzyme shares a significant sequence homology to V8 protease and epidermolytic toxins which are well documented staphylococcal virulence factors. SplA has a very narrow substrate specificity apparently imposed by the precise recognition of three amino acid residues positioned N-terminal to the hydrolysed peptide bond. To explain determinants of this extended specificity we resolve the crystal structure of SplA and define the consensus model of substrate binding. Furthermore we demonstrate that artificial N-terminal elongation of mature SplA mimicking a naturally present signal peptide abolishes enzymatic activity. The probable physiological role of the process is discussed. Of interest, even though precise N-terminal trimming is a common regulatory mechanism among S1 family enzymes, the crystal structure of SplA reveals novel significantly different mechanistic details.
- Department of Analytical Biochemistry, Faculty of Biochemistry, Biophysics and Biotechnology, Jagiellonian University, 30-387 Krakow, Poland.
Organizational Affiliation: