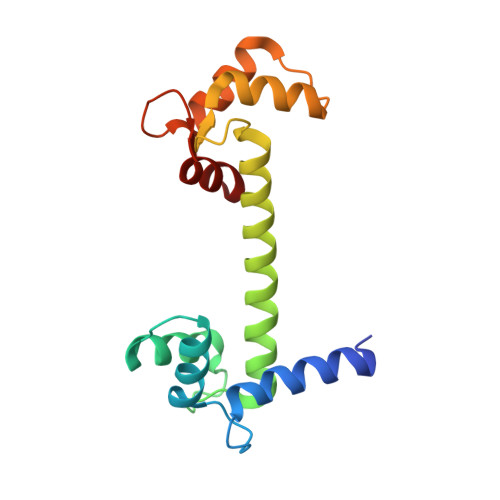
Domain Swapping and Different Oligomeric States for the Complex between Calmodulin and the Calmodulin-Binding Domain of Calcineurin A
Majava, V., Kursula, P.(2009) PLoS One 4: E5402
- PubMed: 19404396
- DOI: https://doi.org/10.1371/journal.pone.0005402
- Primary Citation of Related Structures:
2W73 - PubMed Abstract:
Calmodulin (CaM) is a ubiquitously expressed calcium sensor that engages in regulatory interactions with a large number of cellular proteins. Previously, a unique mode of CaM target recognition has been observed in the crystal structure of a complex between CaM and the CaM-binding domain of calcineurin A. We have solved a high-resolution crystal structure of a complex between CaM and the CaM-binding domain of calcineurin A in a novel crystal form, which shows a dimeric assembly of calmodulin, as observed before in the crystal state. We note that the conformation of CaM in this complex is very similar to that of unliganded CaM, and a detailed analysis revels that the CaM-binding motif in calcineurin A is of a novel '1-11' type. However, using small-angle X-ray scattering (SAXS), we show that the complex is fully monomeric in solution, and a structure of a canonically collapsed CaM-peptide complex can easily be fitted into the SAXS data. This result is also supported by size exclusion chromatography, where the addition of the ligand peptide decreases the apparent size of CaM. In addition, we studied the energetics of binding by isothermal titration calorimetry and found them to closely resemble those observed previously for ligand peptides from CaM-dependent kinases. Our results implicate that CaM can also form a complex with the CaM-binding domain of calcineurin in a 1 ratio 1 stoichiometry, in addition to the previously observed 2 ratio 2 arrangement in the crystal state. At the structural level, going from 2 ratio 2 association to two 1 ratio 1 complexes will require domain swapping in CaM, accompanied by the characteristic bending of the central linker helix between the two lobes of CaM.
- Department of Biochemistry, University of Oulu, Oulu, Finland.
Organizational Affiliation: