Structure and Function of a Complex between Chorismate Mutase and Dahp Synthase: Efficiency Boost for the Junior Partner.
Sasso, S., Okvist, M., Roderer, K., Gamper, M., Codoni, G., Krengel, U., Kast, P.(2009) EMBO J 28: 2128
- PubMed: 19556970
- DOI: https://doi.org/10.1038/emboj.2009.165
- Primary Citation of Related Structures:
2VKL, 2W19, 2W1A - PubMed Abstract:
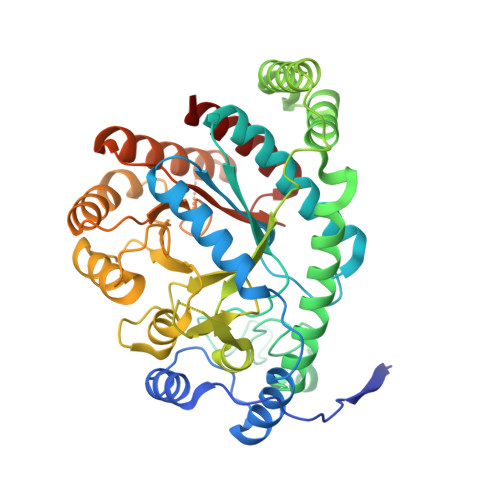
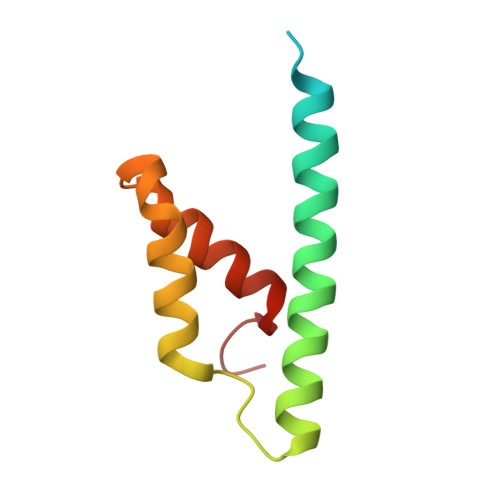
Chorismate mutase catalyzes a key step in the shikimate biosynthetic pathway towards phenylalanine and tyrosine. Curiously, the intracellular chorismate mutase of Mycobacterium tuberculosis (MtCM; Rv0948c) has poor activity and lacks prominent active-site residues. However, its catalytic efficiency increases >100-fold on addition of DAHP synthase (MtDS; Rv2178c), another shikimate-pathway enzyme. The 2.35 A crystal structure of the MtCM-MtDS complex bound to a transition-state analogue shows a central core formed by four MtDS subunits sandwiched between two MtCM dimers. Structural comparisons imply catalytic activation to be a consequence of the repositioning of MtCM active-site residues on binding to MtDS. The mutagenesis of the C-terminal extrusion of MtCM establishes conserved residues as part of the activation machinery. The chorismate-mutase activity of the complex, but not of MtCM alone, is inhibited synergistically by phenylalanine and tyrosine. The complex formation thus endows the shikimate pathway of M. tuberculosis with an important regulatory feature. Experimental evidence suggests that such non-covalent enzyme complexes comprising an AroQ(delta) subclass chorismate mutase like MtCM are abundant in the bacterial order Actinomycetales.
- Laboratory of Organic Chemistry, ETH Zurich, CH-8093 Zurich, Switzerland.
Organizational Affiliation: