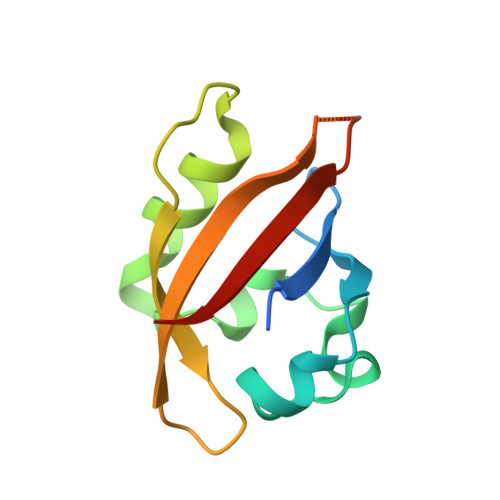
Changes at the Kina Pas-A Dimerization Interface Influence Histidine Kinase Function.
Lee, J., Tomchick, D.R., Brautigam, C.A., Machius, M., Kort, R., Hellingwerf, K.J., Gardner, K.H.(2008) Biochemistry 47: 4051
- PubMed: 18324779
- DOI: https://doi.org/10.1021/bi7021156
- Primary Citation of Related Structures:
2VLG - PubMed Abstract:
The Bacillus subtilis KinA protein is a histidine protein kinase that controls the commitment of this organism to sporulate in response to nutrient deprivation and several other conditions. Prior studies indicated that the N-terminal Per-ARNT-Sim domain (PAS-A) plays a critical role in the catalytic activity of this enzyme, as demonstrated by the significant decrease of the autophosphorylation rate of a KinA protein lacking this domain. On the basis of the environmental sensing role played by PAS domains in a wide range of proteins, including other bacterial sensor kinases, it has been suggested that the PAS-A domain plays an important regulatory role in KinA function. We have investigated this potential by using a combination of biophysical and biochemical methods to examine PAS-A structure and function, both in isolation and within the intact protein. Here, we present the X-ray crystal structure of the KinA PAS-A domain, showing that it crystallizes as a homodimer using beta-sheet/beta-sheet packing interactions as observed for several other PAS domain complexes. Notably, we observed two dimers with tertiary and quaternary structure differences in the crystalline lattice, indicating significant structural flexibility in these domains. To confirm that KinA PAS-A also forms dimers in solution, we used a combination of NMR spectroscopy, gel filtration chromatography, and analytical ultracentrifugation, the results of which are all consistent with the crystallographic results. We experimentally tested the importance of several residues at the dimer interface using site-directed mutagenesis, finding changes in the PAS-A domain that significantly alter KinA enzymatic activity in vitro and in vivo. These results support the importance of PAS domains within KinA and other histidine kinases and suggest possible routes for natural or artificial regulation of kinase activity.
- Department of Biochemistry, UT Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas, Texas 75390-8816, USA.
Organizational Affiliation: