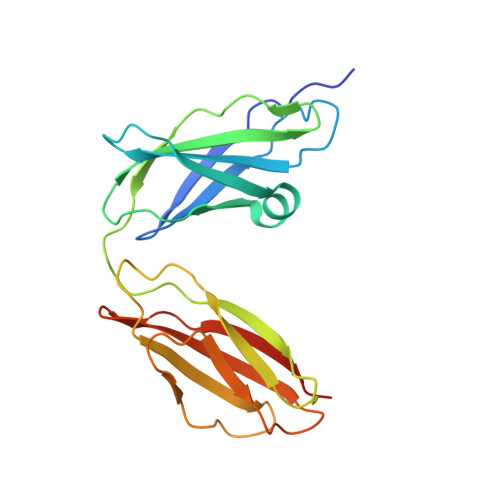
Structure of the Tandem Fibronectin Type 3 Domains of Neural Cell Adhesion Molecule
Carafoli, F., Saffell, J.L., Hohenester, E.(2008) J Mol Biology 377: 524
- PubMed: 18261743
- DOI: https://doi.org/10.1016/j.jmb.2008.01.030
- Primary Citation of Related Structures:
2VKW, 2VKX - PubMed Abstract:
Activation of the fibroblast growth factor receptor (FGFR) by neural cell adhesion molecule (NCAM) is essential for NCAM-mediated neurite outgrowth. Previous peptide studies have identified two regions in the fibronectin type 3 (FN3)-like domains of NCAM as being important for these activities. Here we report the crystal structure of the NCAM FN3 domain tandem, which reveals an acutely bent domain arrangement. Mutation of a non-conserved surface residue (M610R) led to a second crystal form showing a substantially different conformation. Thus, the FN3 domain linker is highly flexible, suggesting that it corresponds to the hinge seen in electron micrographs of NCAM. The two putative FGFR1-binding segments, one in each NCAM FN3 domain, are situated close to the domain interface. They form a contiguous patch in the more severely bent conformation but become separated upon straightening of the FN3 tandem, suggesting that conformational changes within NCAM may modulate FGFR1 activation. Surface plasmon resonance experiments demonstrated only a very weak interaction between the NCAM FN3 tandem and soluble FGFR1 proteins expressed in mammalian cells (dissociation constant >100 muM). Thus, the NCAM-FGFR1 interaction at the cell surface is likely to depend upon avidity effects due to receptor clustering.
- Department of Life Sciences, Biophysics Section, Blackett Laboratory, Imperial College London, London SW7 2AZ, UK.
Organizational Affiliation: