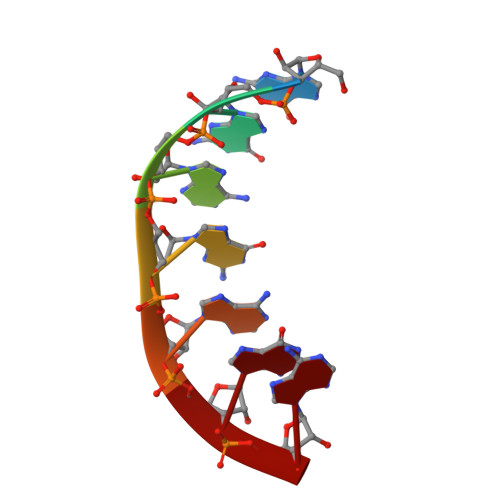
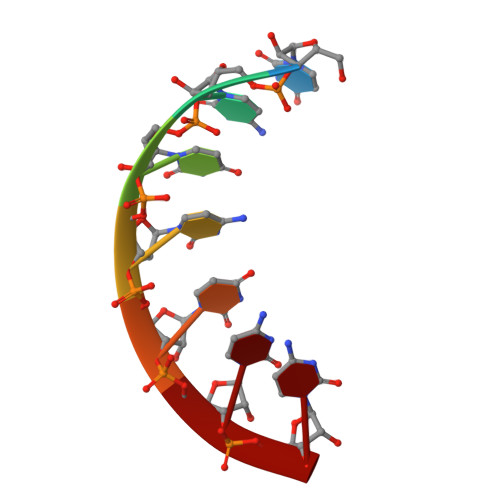
Trnaser Acceptor Stem: Conformation and Hydration of a Microhelix in a Crystal Structure at 1.8 A Resolution.
Forster, C., Brauer, A.B.E., Brode, S., Furste, J.P., Betzel, C., Erdmann, V.A.(2007) Acta Crystallogr D Biol Crystallogr 63: 1154
- PubMed: 18007030
- DOI: https://doi.org/10.1107/S0907444907045271
- Primary Citation of Related Structures:
2V6W - PubMed Abstract:
The crystal structure of a serine-specific tRNA acceptor-stem microhelix, the binding site for the seryl-tRNA synthetase, was solved by X-ray analysis. This seven-base-pair tRNA(Ser) microhelix forms endless rows of helices in the crystal lattice, with two helices stacking 'head-to-head' onto each other, resulting in an intermolecular guanosine stacking of the first purine nucleotides at the 5'-strands of the tRNA(Ser) microhelices. A network of 75 water loci could be associated with each RNA duplex. Unusual local geometric backbone parameters could be detected in the region of the G4 phosphate located in the 5'-strand of the helix, which lead to a ;kink' in this region and to an irregularly bent helix. The role of the specific hydration pattern and of the irregular conformation of the tRNA(Ser) acceptor-stem helix is discussed and summarized.
- Institute of Chemistry and Biochemistry, Free University Berlin, Thielallee 63, 14195 Berlin, Germany.
Organizational Affiliation: