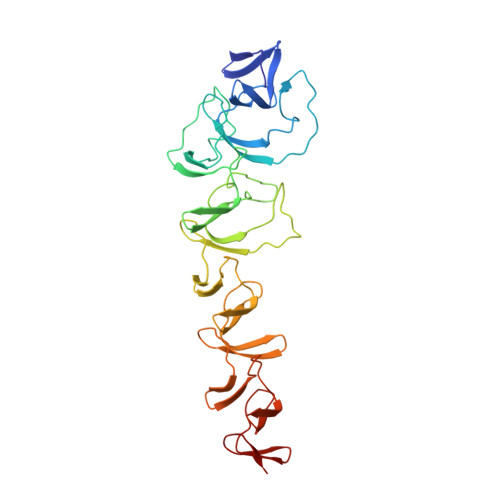
Crystal Structure of Cbpf, a Bifunctional Choline-Binding Protein and Autolysis Regulator from Streptococcus Pneumoniae.
Molina, R., Gonzalez, A., Stelter, M., Perez-Dorado, I., Kahn, R., Morales, M., Campuzano, S., Campillo, N.E., Mobashery, S., Garcia, J.L., Garcia, P., Hermoso, J.A.(2009) EMBO Rep 10: 246
- PubMed: 19165143
- DOI: https://doi.org/10.1038/embor.2008.245
- Primary Citation of Related Structures:
2V04, 2VYU - PubMed Abstract:
Phosphorylcholine, a crucial component of the pneumococcal cell wall, is essential in bacterial physiology and in human pathogenesis because it binds to serum components of the immune system and acts as a docking station for the family of surface choline-binding proteins. The three-dimensional structure of choline-binding protein F (CbpF), one of the most abundant proteins in the pneumococcal cell wall, has been solved in complex with choline. CbpF shows a new modular structure composed both of consensus and non-consensus choline-binding repeats, distributed along its length, which markedly alter its shape, charge distribution and binding ability, and organizing the protein into two well-defined modules. The carboxy-terminal module is involved in cell wall binding and the amino-terminal module is crucial for inhibition of the autolytic LytC muramidase, providing a regulatory function for pneumococcal autolysis.
- Grupo de Cristalografia Macromolecular y Biologia Estructural, Instituto Química-Física Rocasolano, CSIC, Serrano 119, 28006 Madrid, Spain.
Organizational Affiliation: